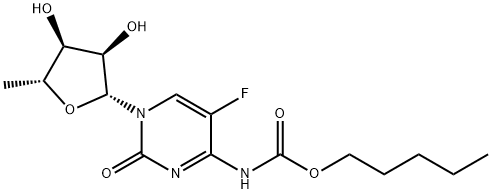
Капецитабин
- английское имяCapecitabine
- CAS №154361-50-9
- CBNumberCB1324457
- ФормулаC15H22FN3O6
- мольный вес359.35
- EINECS604-948-1
- номер MDLMFCD00930626
- файл Mol154361-50-9.mol
химическое свойство
| Температура плавления | 110-121°C |
| плотность | 1.49±0.1 g/cm3(Predicted) |
| Fp | 87℃ |
| температура хранения | 2-8°C |
| растворимость | H2O: soluble10mg/mL, clear (warmed) |
| форма | powder |
| пка | 5.41±0.40(Predicted) |
| цвет | white to beige |
| Мерк | 14,1754 |
| ИнЧИКей | GAGWJHPBXLXJQN-UORFTKCHSA-N |
| SMILES | C[C@H]1O[C@@H](N2C=C(F)C(NC(OCCCCC)=O)=NC2=O)[C@H](O)[C@@H]1O |
| Справочник по базе данных CAS | 154361-50-9(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | capecitabine; Xeloda |
| FDA UNII | 6804DJ8Z9U |
| Словарь наркотиков NCI | capecitabine |
| Код УВД | L01BC06 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T | |||||||||
| Заявления о рисках | 45-60-61-68 | |||||||||
| Заявления о безопасности | 53-22-36/37-45 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | HA3852500 | |||||||||
| кода HS | 29349990 | |||||||||
| Банк данных об опасных веществах | 154361-50-9(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H350:Может вызывать раковые заболевания.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Капецитабин химические свойства, назначение, производство
Описание
Capecitabine is a new oral fluoropyrimidine carbamate for patients with advanced neoplastic disease, approved as Xeloda for the treatment of refractory metastatic breast cancer after failure on Paclitaxel and an anthracycline-based chemotherapy regimen ; it is a prodrug of doxifluridine (5-fluorouracil ; 5-FU) activated by a cascade of 3 enzymes concentrated in human liver and cancer tissue, resulting in the selective release of 5-FU at the tumor site and offering a prolonged tumour exposure to 5-FU. Oral Capecitabine passes intact through the intestinal mucosa, is converted first by carboxylesterase to 5'-deoxy-5- fluorocytidine in the liver, then by cytidine deaminase to 5'-deoxy-5-fluorouridine in the liver and tumour tissues and finally by thymidine phosphorylase to 5-FU in tumors. Therefore, Xeloda is much safer and more effective than 5-FU (for example, in the HCT116 human colon cancer and the MX-1 breast cancer xenograft .models).Химические свойства
Colourless solidИспользование
Capecitabine is an antineoplastic agent. Capecitabine is a prodrug of Doxifluridine (D556750).Определение
ChEBI: A carbamate ester that is cytidine in which the hydrogen at position 5 is replaced by fluorine and in which the amino group attached to position 4 is converted to its N-(penyloxy)carbonyl derivative. Capecitabine is a antineoplastic agen used in the treatment of cancers.Общее описание
The drug is available in a 150- and 500-mg tablets for oraluse. This drug is a fluoropyrimidine carbamate prodrugform of 5-fluorouracil (5-FU). It is used to treat breast cancerand colorectal cancer. The drug is converted to 5-FU bythe enzyme thymidine phosphorylase following esterase activity to hydrolyze the carbamate moiety and deamination.Capecitabine is readily absorbed by the GI tract, and peakplasma levels of 5-FU occur about 2 hours after oral administration.Indications, drug interactions, and toxicities areequivalent to those of 5-FU.Клиническое использование
Capecitabine is indicated for use as first-line therapy in patients with colorectal cancer. It also is used alone or in combination with docetaxel in patients with metastatic breast cancer who have experienced disease progression or recurrence after anthracycline therapy. Given b.i.d. in tablet form, the total daily dose is calculated based on patient body surface area and is taken 30 minutes after eating to avoid food-induced decreases in absorption. In addition to bone marrow suppression, nausea, and vomiting, the drug can induce severe diarrhea and a potentially disabling disorder termed “hand-and-foot syndrome” (palmar-plantar erythrodysethesia). Capecitabine inhibits CYP2C9 and, along with competition for serum protein binding sites, results in clinically significant drug–drug interactions with both warfarin and phenytoin.Метаболизм
Although capecitabine is a carbamylated analogue of cytidine , the drug actually is another 5-fluoro-dUMP prodrug. Given orally, it is extensively metabolized to fluorouracil, which is then converted to the active fluorinated deoxyribonucleotide as previously described. Thymidine phosphorylase, an enzyme involved in this biotransformation, is much more active in tumors than in normal tissue, which improves the tumor-selective generation of fluorouracil. Levels of active drug in the tumor can be up to 3.5-fold higher than in surrounding tissue, leading to a lower incidence of side effects compared to fluorouracil therapy. Because capecitabine is biotransformed to fluorouracil, it follows the same catabolic and elimination pathways reported for 5-fluorouracil.Капецитабин запасные части и сырье
Капецитабин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0531-69954981 +8615666777973 |
China | 211 | 58 | ||
| +86-0571-85134551 | China | 15352 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +86-53169958659 +86-13153181156 |
China | 294 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 |