
Hexaphenylbenzene
- английское имяHexaphenylbenzene
- CAS №992-04-1
- CBNumberCB8389989
- ФормулаC42H30
- мольный вес534.69
- EINECS213-591-7
- номер MDLMFCD00003057
- файл Mol992-04-1.mol
химическое свойство
| Температура плавления | >300 °C (lit.) |
| Температура кипения | 547.47°C (rough estimate) |
| плотность | 0.8530 (rough estimate) |
| показатель преломления | 1.4690 (estimate) |
| форма | powder to crystal |
| цвет | White to Light yellow |
| Растворимость в воде | Insoluble in water. |
| БРН | 1895903 |
| FDA UNII | 35L5JAJ94N |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
| Заявления о безопасности | 24/25-25-24 | |||||||||
| WGK Германия | 3 | |||||||||
| кода HS | 29029090 | |||||||||
| NFPA 704: |
|
Hexaphenylbenzene химические свойства, назначение, производство
Химические свойства
white to off-tan powderИспользование
Hexaphenylbenzene was used to prepare the fluorescent nanorods used for the detection of trinitrotoulene (TNT).прикладной
Hexaphenylbenzene can be used as a starting material to synthesize:1,2,3,4,5,6-Hexacyclohexylcyclohexane by Pd/C catalyzed hydrogenation reaction.
Stable hexatrityl cations and porous organic polymers for applications in catalysis and gas storage.
Hexa-peri-hexabenzocoronene via one-pot substitution and oxidative cyclodehydrogenation reaction in the presence of t-BuCl/FeCl3.
as a structural unit for the synthesis of polymers of intrinsic microporosity.
Синтез
Hexaphenylbenzene has been prepared by heating tetraphenylcyclopentadienone and diphenylacetylene without solvent and by trimerization of diphenylacetylene with bis-(benzonitrile)-palladium chloride and other catalysts. The reaction proceeds via a Diels-Alder reaction to give the hexaphenyldienone, which then eliminates carbon monoxide.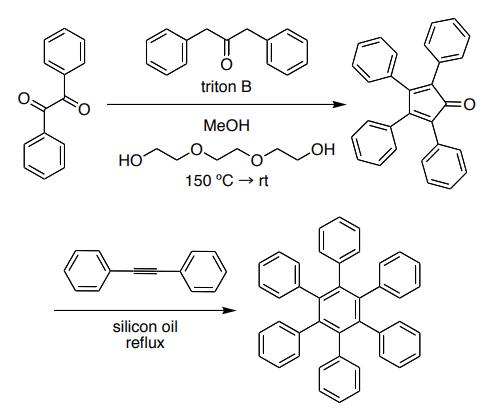
Multi-Step Synthesis of hexaphenylbenzene from benzil
Procedure: Add 0.8 g of tetraphenylcyclopentadienone, 0.8 g of diphenylacetylene (synthesized by you in CH 2270 lab last semester), and 4 g of benzophenone to a 25 mL round-bottom flask. Place a magnetic stir bar in the flask. Make sure no material lodges on the neck or walls of the flask. Attach the condenser to the round-bottom flask. Do not attach the hoses. The condenser will be used as an “air condenser” for this experiment. Heat the reaction mixture VERY HIGH with the sand bath on the hot plate/stirrer. Benzophenone is your solvent and its boiling point is over 300 °C! Heat the reaction mixture to reflux. Carbon monoxide is evolved, the purple color begins to fade in 15-20 minutes, and the color changes to a reddish brown in 25-30 minutes. When no further lightening in color is observed (after about 45 minutes), the heat is removed and 1 mL of diphenyl ether is added to prevent subsequent solidification of the benzophenone. The crystals that separate are brought into solution by reheating and the solution is let stand for crystallization at room temperature. The product is collected and washed free of brown solvent with toluene to give colorless plates.
Hexaphenylbenzene поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +undefined-21-51877795 | China | 32965 | 60 | |
| 0431-80514535 13634302652 | CHINA | 967 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| 021-64478606 +8615900664856 |
China | 2062 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-0571-85585865 +8613336024896 |
China | 19183 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| +1-817863-6948 +1-(817)863-6948 |
United States | 19632 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +undefined18143011203 | China | 42056 | 58 |