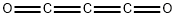
диоксид трикарбона
- английское имяtricarbon dioxide
- CAS №504-64-3
- CBNumberCB81268795
- ФормулаC3O2
- мольный вес68.03
- файл Mol504-64-3.mol
химическое свойство
| Температура плавления | -111.3° |
| Температура кипения | bp760 6.8° |
| плотность | d40 1.114 |
| показатель преломления | n0D 1.45384; nD-12 1.46757 |
| растворимость | reacts with H2O |
| форма | colorless gas |
| цвет | colorless |
| Растворимость в воде | forms malonic acid with H2O [MER06] |
| FDA UNII | 9M2M4H5WEJ |
диоксид трикарбона химические свойства, назначение, производство
Химические свойства
colorless, highly refractive liquid or colorless gas, which burns with a blue sooty flame; odor like acrolein or mustard oil; structure: O=C=C=C=O [MER06]Физические свойства
Colorless gas; strong, pungent odor; gas density 2.985 g/L; liquid density 1.114 g/mL at 0°C; refractive index 1.4538 (at 0°C); vapor pressure 588 torr at 0°C; liquefies at 6.8°C; freezes at -111.3°C; burns with a blue sooty flame; reacts with water. The compound is unstable, polymerizing on storage.Использование
preparation of malonates; improving dye affinity of fibers.Подготовка
Carbon suboxide is prepared by dehydration of malonic acid with phosphorus pentoxide in vacuum at 140 to 150°C:CH2(COOH)2→ C3O2 + 2H2O
Alternatively, the compound may be prepared by thermal dissociation of diacetyltartaric anhydride.
Опасность
Carbon suboxide forms explosive mixtures in air. The lower and upper explosive limits are 6 to 30% by volume in air, respectively. The gas is a strong lacrimator and an irritant to eyes, nose and respiratory tract. Exposure to high concentations is dangerous. .