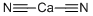
ЦИАНИД КАЛЬЦИЯ
- английское имяCALCIUM CYANIDE
- CAS №592-01-8
- CBNumberCB8105338
- ФормулаC2CaN2
- мольный вес92.11
- EINECS209-740-0
- номер MDLMFCD00049398
- файл Mol592-01-8.mol
| Температура плавления | 640 estimated [KIR78] |
| плотность | 1.8 g/cm3 |
| растворимость | soluble in H2O, ethanol |
| форма | white rhombohedral crystals |
| цвет | white rhomb crystals, crystalline; hygroscopic |
| Растворимость в воде | soluble H2O, gradually releasing HCN [MER06] |
| Пределы воздействия | TLV-TWA (measured as CN) skin 5 mg(CN)/ m3 (ACGIH); 5 mg(CN)/m3/10-min ceiling (NIOSH). |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | 07DFJ2NTDD |
| Система регистрации веществ EPA | Calcium cyanide (592-01-8) |
| Коды опасности | T+,N | |||||||||
| Заявления о рисках | 28-32-50/53 | |||||||||
| Заявления о безопасности | 7/8-23-36/37-45-60-61 | |||||||||
| РИДАДР | 1575 | |||||||||
| Класс опасности | 6.1(a) | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 28371990 | |||||||||
| Банк данных об опасных веществах | 592-01-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 39 mg/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H300:Смертельно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P391:Ликвидировать просыпания/проливы/утечки.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ЦИАНИД КАЛЬЦИЯ химические свойства, назначение, производство
Описание
Calcium cyanide is used mainly for the extraction or cyanidation of gold and silver ores. It is also used in the production of prussiates or ferrocyanides, in the froth flotation of minerals, in processes where gold complexes are adsorbed on carbon, in the manufacture of stainless steel, as a fumigant and rodenticide, and as a cement stabiliser. The main users of cyanides are the steel, electroplating, mining, and chemical industries. The principal cyanide compounds used in industrial operations are potassium and sodium cyanide and calcium cyanide, particularly in metal leaching operations. Cyanides have been well established in uses as insecticides and fumigants; in the extraction of gold and silver ores; in metal cleaning; in the manufacture of synthetic fibres, various plastics, dyes, pigments, and nylon; and as reagents in analytical chemistry. Calcium cyanide decomposes on heating above 350°C, producing toxic fumes including nitrogen oxides and HCN. It reacts violently with water, moist air, carbon dioxide, acids, and acid salts producing highly toxic and flammable HCN. It reacts violently when heated with oxidising substances causing fire and explosion hazard.Химические свойства
Calcium cyanide is a white crystalline solid or powder. Odor of hydrogen cyanide.Использование
Calcium cyanide is used for the extraction of gold and silver from their ores, in the froth flotation of minerals, as a fumigant, and as a rodenticide.Методы производства
Calcium cyanide is made commercially from lime, calcium oxide, coke, and nitrogen. The reactions are carried out in an electric furnace. The resulting melt is cooled rapidly to prevent reversion to calcium cyanamide. The product is marketed in the form of flakes, which are dark gray because of the presence of carbon. The extraction or cyanidation of precious-metal ores was the first and is still the largest use for calcium cyanide.Общее описание
White crystals or powder or gray-black powder (technical grade). Toxic by skin absorption through open wounds, by ingestion, and by inhalation.Реакции воздуха и воды
Water soluble with evolution of some hydrogen cyanide, a flammable poison gas. Release of gas is much more rapid if acid is present.Профиль реактивности
CALCIUM CYANIDE gives weakly acidic solutions. Contact with acids causes rapid evolution of hydrogen cyanide. Incompatible with isocyanates, nitrides, and peroxides. May react rapidly with oxidizing agents.Опасность
Toxic by ingestion and skin absorption.Угроза здоровью
Calcium cyanide is a highly poisonous compound to humans, animals, and fish. The toxic routes are ingestion, skin contact, and inhalation of the dust. It forms HCN readily when it reacts with CO2 or water. This makes it highly hazardous, more so than the alkalimetal cyanides, although the LD50 value of Ca(CN)2 is greater than the sodium or potassium cyanides.LD50 value, oral (rats): 39 mg/kg.
Пожароопасность
Special Hazards of Combustion Products: Decomposes in fire to give very toxic gases, including hydrogen cyanide.Профиль безопасности
A deadly poison by ingestion and probably other routes. When heated to decomposition it emits toxic fumes of NO, and CN-. See also CALCIUM COMPOUNDS and CYANIDEВозможный контакт
Calcium cyanide is used as a fumigant; as a rodenticide; in leaching precious metal ores; in the manufacture of stainless steel; and as a stabilizer forcement. Used as raw material for production of nitrogenous compounds and in treatment of alcoholismхранилище
Calcium cyanide is stored in tight containers free from moisture. Proper ventilation and protective equipment should be used while handling the solid or while preparing an aqueous solution. It is shipped in mild-steel or fiber drums.Перевозки
UN1575 Calcium cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materialsНесовместимости
Contact with water, acids, acidic salts; moist air, or carbon dioxide, forms highly toxic and flammable hydrogen cyanide. Incompatible with fluorine, magnesium. Reacts violently when heated with nitrites, nitrates, chlorates, and perchlorates. Calcium cyanide decomposes in high heat forming hydrogen cyanide and nitrous oxides fumesУтилизация отходов
Add cyanide waste to strong alkaline sodium hypochlorite. Let stand 24 hours then flush to sewage plant.