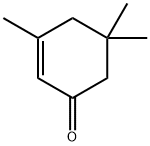
Изофорон
- английское имяIsophorone
- CAS №78-59-1
- CBNumberCB7743348
- ФормулаC9H14O
- мольный вес138.21
- EINECS201-126-0
- номер MDLMFCD00001584
- файл Mol78-59-1.mol
| Температура плавления | -8 °C (lit.) |
| Температура кипения | 213-214 °C (lit.) |
| плотность | 0.923 g/mL at 25 °C (lit.) |
| плотность пара | 4.77 (vs air) |
| давление пара | 0.2 mm Hg ( 20 °C) |
| показатель преломления | n |
| FEMA | 3553 | ISOPHORONE |
| Fp | 184 °F |
| температура хранения | Store below +30°C. |
| растворимость | 14.5g/l |
| форма | Liquid |
| цвет | Clear colorless to yellow |
| Запах | Like camphor. |
| Пределы взрываемости | 0.8-3.8%(V) |
| Odor Type | woody |
| Биологические источники | synthetic |
| Вязкость | 2.83mm2/s |
| Растворимость в воде | Soluble in water (12g/L). |
| Мерк | 14,5196 |
| Номер JECFA | 1112 |
| БРН | 1280721 |
| констант закона Генри | (x 10-6 atm?m3/mol): 5.8 (calculated, U.S. EPA, 1980a) |
| Пределы воздействия | TLV-TWA 25 mg/m3 (5 ppm); IDLH 800 ppm. |
| Стабильность | Stable. Substances to be avoided include strong bases, strong acids and strong oxidizing agents. |
| LogP | 1.67-1.7 at 20℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | ISOPHORONE |
| FDA 21 CFR | 175.105 |
| Справочник по базе данных CAS | 78-59-1(CAS DataBase Reference) |
| FDA UNII | 2BR99VR6WA |
| Справочник по химии NIST | 2-Cyclohexen-1-one, 3,5,5-trimethyl-(78-59-1) |
| Система регистрации веществ EPA | Isophorone (78-59-1) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 21/22-36/37-40 | |||||||||
| Заявления о безопасности | 13-23-36/37/39-46 | |||||||||
| РИДАДР | UN 3082 9 / PGIII | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 4 ppm (23 mg/m3) | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | GW7700000 | |||||||||
| Температура самовоспламенения | 864 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2914 29 00 | |||||||||
| Банк данных об опасных веществах | 78-59-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in male, female rats and male mice (mg/kg): 2700 ±200, 2100 ±200, 2200 ±200 orally (PB90-180225) | |||||||||
| ИДЛА | 200 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H302+H312:Вредно при проглатывании или при попадании на кожу.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Изофорон химические свойства, назначение, производство
Химические свойства
Isophorone is a colorless low volatility liquid that smells like peppermint. It is miscible with most organic solvents and can dissolve 1.2g in 100g of water. When exposed to light, it becomes a dimer, which is oxidized in air to generate 4,4,6-Trimethyl-1,2-cyclohexanedione.Физические свойства
Clear, colorless liquid with a sharp peppermint or camphor-like odor. Experimentally determined detection and recognition odor threshold concentrations were 1.1 mg/m3 (190 ppbv) and 3.0 μg/m3 (530 ppbv), respectively (Hellman and Small, 1974).Вхождение
Reported found in Burley tobacco, cranberry, macadamia nuts, peas, roasted filbert, saffron, wine, osmanthus, grapefruit juice, papaya, kohlrabi, Parmesan cheese, roast beef, black tea, oats, Japanese plum, prunes, plumcot, starfruit, mango, rice, buckwheat, okra and sweet grass oil.Использование
Isophorone is used as a solvent for vinylresins and cellulose esters, and in pesticides. solvent in some printing inks, paints, lacquers and adhesives. As a cyclic unsaturated ketone, isophorone can be aromatized to 3,5-dimethylphenol or 2,3,5-trimethylphenol. Hydrogenation leads, depending on conditions, to 3,3,5-trimethylcyclohexanone or 3,3,5- trimethylcyclohexanol, from which trimethyladipic acid is obtained by oxidation [78]. Trimethyladipic acid is used for the production of 2,2,4- trimethylhexamethylenediamine, which is a precursor for polyamides and polyurethanes. Addition of hydrogen cyanide to the olefinic double bond, followed by amination of the keto group in the presence of hydrogen, gives 3,5,5-trimethyl-3-aminomethylcyclohexylamine [2855-13-2], isophorone diamine. Reaction of the latter with phosgene gives isophorone diisocyanate [4098-71-9]. Both compounds are used in large quantities for the production of polymers, mainly polyurethanes. Worldwide production capacity for isophorone is ca. 50 000 t/a.Определение
ChEBI: Isophorone is a cyclic ketone, the structure of which is that of cyclohex-2-en-1-one substituted by methyl groups at positions 3, 5 and 5. It has a role as a solvent and a plant metabolite. It is a cyclic ketone and an enone.Подготовка
Isophorone is produced on a multi-thousand ton scale by the aldol condensation of acetone using KOH. Diacetone alcohol, mesityl oxide, and 3-hydroxy-3,5,5-trimethylcyclohexan-1-one are intermediates. A side product is beta-isophorone, where the C=C group is not conjugated with the ketone.Общее описание
A clear colorless liquid, with a camphor-like odor. Less dense than water and insoluble in water. Boiling point 420°F. Flash point near 200°F. Contact irritates skin and eyes. Toxic by ingestion. Used as a solvent and in pesticides.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Ketones, such as Isophorone, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. Forms explosive peroxidesУгроза здоровью
Isophorone is an irritant, moderately toxic athigh concentrations, mutagenic and possiblycarcinogenic. Inhalation of its vapors cancause mild irritation of the eyes, nose, andthroat. Exposure to 840 ppm for 4 hours resulted in severe eye irritation in guineapigs. Its irritation effect on human eyes maybe felt at 25–40 ppm. At concentrationsabove 200 ppm, it may cause irritation ofthe throat, headache, nausea, dizziness, and afeeling of suffocation (ACGIH 1986). In rats,exposure to 1840 ppm for 4 hours was fatal.Ingestion of isophorone can cause narcosis,dermatitis, headache, and dizziness.LD50 value, oral (rats): 2330 mg/kg
Isophorone is mutagenic and when fed torats orally, 258 g/kg for 2 years, it causedkidney tumor. Its carcinogenicity on humansis not reported.
Пожароопасность
Combustible.Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Промышленное использование
Isophorone is an excellent solvent for cellulose esters, nitrocellulose, natural and synthetic resins. Isophorone has a very high aromatic hydrocarbon dilution ratio in nitrocellulose formulations: 5.7 for toluene and 5.1 for xylene. The excellent solvency of isophorone allows the preparation of 45% solids nitrocellulose solutions at room temperatures.Канцерогенность
In a 2 year bioassay in rats and mice (conducted by gavage on a 5- day/week treatment schedule) at dose levels of 0, 250, and 500 mg/kg/day isophorone, there was decreased survival of male rats and slight nephrotoxicity in female rats. There was no evidence of carcinogenicity in female rats or mice. In male rats, there was an increase in renal tumors in animals given either 250 or 500 mg/kg/day, and a low incidence of preputial gland tumors at 500 mg/kg. Other proliferative lesions in male rats included hyperplasia of the renal pelvis and tubular cell hyperplasia.Экологическая судьба
Biological. The pure culture Aspergillus niger biodegraded isophorone to 3,5,5-trimethyl-2- cyclo-hexene-1,4-dione, 3,5,5-trimethylcyclohexane-1,4-dione, (S)-4-hydroxy-3,5,5-trimethyl-2- cyclohex-1-one, and 3-hydroxymethyl-5,5-dimethyl-2-cyclohexen-1-one (Mikami et al., 1981).Chemical/Physical. At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent concentration of 34 mg/L. The adsorbability of the carbon used was 193 mg/g carbon (Guisti et al., 1974).
Isophorone will not hydrolyze in water.
At influent concentrations of 10, 1.0, 0.1, and 0.001 mg/L, the GAC adsorption capacities were 78.3, 32.0, 13.1, and 5.4 mg/g, respectively (Dobbs and Cohen, 1980).
Методы очистки
Wash isophorone with aqueous 5% Na2CO3 and then distil it under reduced pressure immediately before use. Alternatively, it can be purified via the semicarbazone. [Erskine & Waight J Chem Soc 3425 1960, Beilstein 7 IV 165.]Утилизация отходов
Incineration.Изофорон запасные части и сырье
Изофорон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +8615530197691 | China | 105 | 58 |