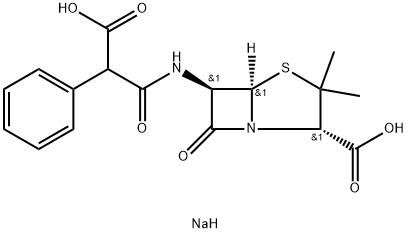
Carbenicillin динатриевой сол
- английское имяCarbenicillin disodium
- CAS №4800-94-6
- CBNumberCB7308325
- ФормулаC17H19N2NaO6S
- мольный вес402.4
- EINECS225-360-8
- номер MDLMFCD00077683
- файл Mol4800-94-6.mol
химическое свойство
| Температура плавления | >190°C (dec.) |
| альфа | +175~+190°(D/20℃)(c=4,H2O)(calculated on the dehydrous basis) |
| температура хранения | Inert atmosphere,2-8°C |
| растворимость | H2O: 50 mg/mL |
| форма | powder |
| цвет | white to off-white |
| РН | 5.5~7.5 (10g/l, 25℃) |
| Растворимость в воде | Soluble in water. |
| Мерк | 13,1801 |
| БРН | 5722128 |
| ИнЧИКей | HUKAYYNGXWHSGG-MRDJHEKFNA-N |
| SMILES | C([C@H]1C(S[C@]2([H])[C@H](NC(=O)C(C3C=CC=CC=3)C(=O)O)C(=O)N12)(C)C)(=O)O.[NaH] |&1:1,4,6,r| |
| FDA UNII | 9TS4B3H261 |
| UNSPSC Code | 51282413 |
| Коды опасности | Xn |
| Заявления о рисках | 42/43 |
| Заявления о безопасности | 22-36/37-36 |
| РИДАДР | UN 1170 3/PG 3 |
| WGK Германия | 2 |
| RTECS | ON9105000 |
| F | 3-8-10 |
| кода HS | 29411000 |
| Токсичность | LD50 i.p. in rats: >2000 mg/kg (Goldenthal) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
Carbenicillin динатриевой сол химические свойства, назначение, производство
Описание
Carbenicillin is a broad-spectrum carboxypenicillin antibiotic. It is active against Gram-negative and certain Gram-positive bacteria, including S. pyogenes, S. epidermidis, P. mirabilis, P. vulgaris, E. coli, and P. aeruginosa (MICs = 0.19, 1.56, 1.56, 3.12, 3.12, and 50 μg/ml, respectively). It is also active against penicillinase-producing and non-producing strains of S. aureus (MICs = 1.56 and 12.5 μg/ml, respectively). Carbenicillin is protective against systemic S. pyogenes, P. vulgaris, E. coli, and S. aureus infection in a mouse model of systemic lethal infection with 50% protective dose (PD50) values of 7.8, 224, 19.3, and 34 mg/kg, respectively. It also decreases viable colony counts in the kidney in a rat model of P. vulgaris or E. coli urinary tract infection when administered at a dose of 100 mg/kg. Formulations containing carbenicillin have previously been used in the treatment of upper and lower urinary tract infections and prostatitis.Химические свойства
white PowderИспользование
Carbenicillin disodium salt is a water-soluble antibiotic which is effective against gram-negative bacteria.Carbenicillin disodium salt is widely utilized as an antibiotic which is used to control bacterial cell-wall synthesis by inactivating transpeptidases on the inner surface of the bacterial cell membrane. It has been involved in the study of the role of penicillin-sensitive transpeptidases in cell wall biosynthesis.Общее описание
Carbenicillin was synthesized by Brain et al. of Beecham Research Laboratories in 1965. It was the first synthetic penicillin to show activity against Pseudomonas aeruginosa. Although its activity against the microorganism is not strong (MIC = 25 – 100 μg/mL), it is widely used against P. aeruginosa infections because of its low toxicity and the lack of other antibiotics suitable for use against this microorganism. Carbenicillin is mainly used clinically to treat urinary tract and respiratory tract infections and sepsis caused by Proteus, Escherichia coli, Klebsiella, and Pseudomonas aeruginosa.Клиническое использование
Carbenicillin disodium, disodium α-carboxybenzylpenicillin(Geopen, Pyopen), is a semisynthetic penicillin releasedin the United States in 1970, which was introduced inEngland and first reported by Ancred et al. in 1967.Examination of its structure shows that it differs from ampicillinin having an ionizable carboxyl group rather than anamino group substituted on the α-carbon atom of the benzylside chain. Carbenicillin has a broad range of antimicrobialactivity, broader than any other known penicillin, a propertyattributed to the unique carboxyl group. It has been proposedthat the carboxyl group improves penetration of themolecule through cell wall barriers of Gram-negativebacilli, compared with other penicillins.Carbenicillin is not stable in acids and is inactivated bypenicillinase. It is a malonic acid derivative and, as such, decarboxylatesreadily to penicillin G, which is acid labile.Solutions of the disodium salt should be freshly prepared but,when refrigerated, may be kept for 2 weeks. It must be administeredby injection and is usually given intravenously.
Carbenicillin has been effective in the treatment of systemicand urinary tract infections caused by P. aeruginosa,indole-producing Proteus spp., and Providencia spp., all ofwhich are resistant to ampicillin. The low toxicity of carbenicillin,with the exception of allergic sensitivity, permits theuse of large dosages in serious infections. Most cliniciansprefer to use a combination of carbenicillin and gentamicinfor serious pseudomonal and mixed coliform infections. Thetwo antibiotics are chemically incompatible, however, andshould never be combined in an intravenous solution.
Carbenicillin динатриевой сол запасные части и сырье
Carbenicillin динатриевой сол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618762124502 | China | 27 | 58 | ||
| +8617732866630 | China | 8773 | 58 | ||
| +8613288715578 | China | 12554 | 58 | ||
| +86-13131129325 | China | 5251 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21622 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29855 | 58 | ||
| 18627774460 | CHINA | 742 | 58 | ||
| +8618523575427 | China | 49730 | 58 | ||
| 18503026267 | CHINA | 9636 | 58 | ||
| +8618530059196 | China | 12394 | 58 |