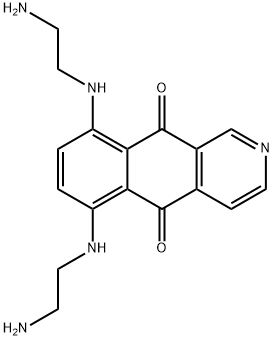
Пиксантрон
- английское имяPixantrone
- CAS №144510-96-3
- CBNumberCB71321822
- ФормулаC17H19N5O2
- мольный вес325.37
- номер MDLMFCD09837694
- файл Mol144510-96-3.mol
химическое свойство
| Температура плавления | >173°C (dec.) |
| Температура кипения | 650.0±55.0 °C(Predicted) |
| плотность | 1.405 |
| температура хранения | Refrigerator |
| растворимость | DMSO (Slightly), Methanol (Slightly) |
| форма | Solid |
| пка | 9.34±0.10(Predicted) |
| цвет | Dark Blue to Very Dark Blue |
| Словарь онкологических терминов NCI | pixantrone |
| FDA UNII | F5SXN2KNMR |
| Словарь наркотиков NCI | pixantrone dimaleate |
| Код УВД | L01DB11 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Пиксантрон химические свойства, назначение, производство
Описание
In May 2012, pixantrone was approved by the European Commission as a single agent for the treatment of relapsed or refractory aggressive B-cell non-Hodgkin lymphoma (NHL) in adult patients who have failed on at least two previous therapies. Pixantrone (also known as BBR- 2778) is an anthracycline analogue that was specifically designed to address the cardiotoxicity seen in earlier agents by replacement of a 1,4- dihydroxyanthracene-9,10-dione core with a benzoisoquinoline-5,10-dione ring system. Pixantrone inhibits topoisomerase II by intercalation with DNA and is also believed to form covalent adducts with the N-2 amino group of guanine via a formaldehyde aminal formed with the primary amino groups. Pixantrone is less cytotoxic than other anthracycline derivatives, but shows good antitumor activity in vivo in a variety of preclinical tumor models, including leukemia and lymphoma models. Pixantrone also demonstrated significantly reduced cardiotoxicity in preclinical models compared with the anthracyclines doxorubicin and mitroxantrone. Pixantrone was synthesized by Friedel–Crafts reaction of pyridine-3,4-dicarboxylic acid anhydride with 1,4-difluorobenzene to give a ketoacid that was cyclized to the tricyclic core by treatment with fuming sulfuric acid at 140℃. Reaction of the resulting 6,9-difluorobenzoisoquinoline-5-10-dione with ethylenediamine followed by careful pH adjustment and treatment with maleic acid gave pixantrone in good overall yield and purity.Использование
Pixantrone is an antineoplastic drug belonging to group of antitumor antibiotics. Pixantrone is an anlogue of Mitoxantrone (M373425) and is just as potent in the treatment of multiple sclerosis with fewer toxic effects on cardiac tissue. Studies suggest that Pixantrone significantly reduces amyloid beta (A beta(1-42)) neurotoxicity, a mechanism implicated in Alzheimer's disease.Клиническое использование
Pixuvri (Pixantrone dimaleate) is a novel aza-anthracenedione derivative approved in Europe for the treatment of adult patients with non-Hodgkin B-cell lymphoma. It is also being pursued as a treatment for various cancers, and specifically as an alternative to other structurally-related drugs like mitoxantrone, employed for treatment of breast cancer, acute myeloid leukemia (AML), and non- Hodgkins lymphoma.Pixantrone dimaleate has been designed to maintain antitumor efficacy while decreasing highly cardiotoxic side effects observed during treatment with other related anti-tumor anthracenedione derivatives. Like many anthracenedione drugs, the mechanism of action for pixantrone dimaleate likely includes a number of pathways and processes, with studies suggesting intercalation into DNA and/or interference with DNA –Topoisomerase II activity, leading to subsequent protein associated-DNA strand breaks and eventually to cell death.Пиксантрон запасные части и сырье
Пиксантрон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| 0086 18039857276 18039857276 |
CHINA | 2783 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +1-2135480471 +1-2135480471 |
China | 10473 | 58 | |
| +1-708-310-1919 +1-13798911105 |
United States | 6391 | 58 | |
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 | |
| +8617327281506 | China | 1519 | 58 |