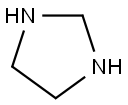
имидазолидин
- английское имяImidazolidine
- CAS №504-74-5
- CBNumberCB71133311
- ФормулаC3H8N2
- мольный вес72.11
- EINECS263-058-8
- номер MDLMFCD19216513
- файл Mol504-74-5.mol
химическое свойство
| Температура плавления | 68.2-68.8 °C |
| Температура кипения | 92.8±8.0 °C(Predicted) |
| плотность | 0.892±0.06 g/cm3(Predicted) |
| температура хранения | Keep in dark place,Inert atmosphere,Room temperature |
| пка | 10.33±0.20(Predicted) |
| FDA UNII | AEE9PL2D22 |
| Система регистрации веществ EPA | Imidazolidine (504-74-5) |
имидазолидин химические свойства, назначение, производство
Использование
The imidazolines, was discovered at American Cyanamid in the early 1980s. Extensive research has led to the development of four commercial compounds: imazapyr, imazamethabenzmethyl, imazethapyr, and imazaquin. Like the sulfonylureas, the imidazolines are extremely active at low rates.Синтез
Imidazolidine is produced by a cyclocondensation reaction between ethylenediamine and an aldehyde. The yield is 70 %. The reaction conditions are that one of the amino groups of ethylenediamine is present using the secondary amine form.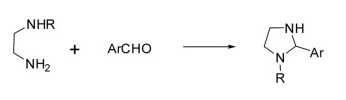
Synthesis of Imidazolidine derivatives including:
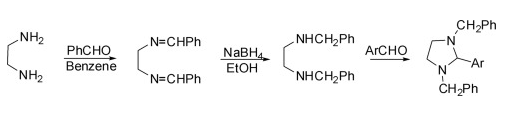
(1) Synthesis of 1,3-dibenzyl-2-arylimidazolidine
It is divided into three steps: the first step is the condensation of ethylenediamine with aldehyde in dry benzene to obtain N,N′-dibenzylidene-1,2-diamine, and the second step is the reduction of N,N′-dibenzylidene ethylenediamine to N,N′-dibenzylidene ethylenediamine in ethanol with sodium borohydride. The substituted diamine was condensed with an aryl aldehyde in the final step to give 1,3-dibenzyl-2-arylimidazolidine.
(2)Synthesis of 2-iminoimidazolidine
Method: Ethylenediamine reacts with cyanobromide to form 2-iminoimidazolidine by substitution-cyclisation.

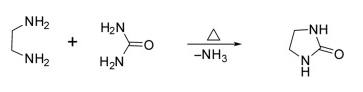
(3) Synthesis of Imidazolidin-2-one
Methods: Imidazolidin-2-one was prepared by heating ethylenediamine and urea with 75% yield.
Структура и конформация
Dihydroimidazole is a five-membered, nonplanar, and nonaromatic heterocycle, derived by the partial reduction of one of the two double bonds of the imidazole ring. The dihydroimidazoles are also referred to as imidazolines and there are three possible regioisomeric forms: 4,5-dihydroimidazole (2-imidazoline), 2,5-dihydroimidazole (3-imidazoline), and 2,3- dihydroimidazole (4-imidazoline). The 2- and 3-imidazolines contain an imine center, while 4-imidazoline contains an alkene substructure. Among these three isomeric forms, the chemistry of 2-imidazoline is more developed than 3- and 4-imidazolines.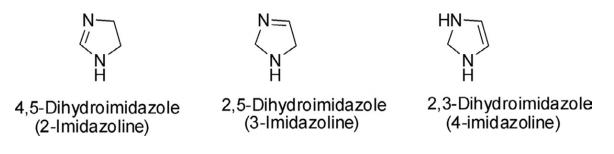
имидазолидин запасные части и сырье
запасной предмет
- amidoaminosurfactans
- Inhibitor
- 2-этил-4-метилимидазол
- 2-(1-нафтилметил)-2-имидазолин гидрохлорид
- Имидаприл
- cocoyl coarboxy methyl imidazoline acetat XCG-CA-2 type
- cmtirust agent T-708
- Имидокарб
- acid washing corrosion inhibitor Sx-1
- corrosion inhibitor 581
1of4
имидазолидин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |