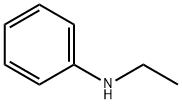
N-Этиланилин
- английское имяN-Ethylaniline
- CAS №103-69-5
- CBNumberCB6852629
- ФормулаC8H11N
- мольный вес121.18
- EINECS203-135-5
- номер MDLMFCD00009025
- файл Mol103-69-5.mol
| Температура плавления | -63 °C |
| Температура кипения | 205 °C(lit.) |
| плотность | 0.963 g/mL at 25 °C(lit.) |
| плотность пара | 4.2 (vs air) |
| давление пара | 0.2 mm Hg ( 25 °C) |
| показатель преломления | n |
| Fp | 185 °F |
| температура хранения | Store below +30°C. |
| растворимость | 2.7g/l |
| форма | Liquid |
| пка | 5.12(at 24℃) |
| цвет | Clear light yellow to light brown |
| РН | 11 (2.7g/l, H2O, 20℃) |
| Пределы взрываемости | 1.8-10.0%(V) |
| Растворимость в воде | 50 g/L (20 ºC) |
| Чувствительный | Air & Light Sensitive |
| Мерк | 14,3764 |
| БРН | 507468 |
| Пределы воздействия | NIOSH: IDLH 100 ppm |
| Диэлектрическая постоянная | 5.9(20℃) |
| Стабильность | Stable, but decomposes upon prolonged exposure to air or light. Combustible. Incompatible with strong oxidizing agents. May react violently with nitric acid. |
| LogP | 2.26 at 25℃ and pH6-8 |
| Справочник по базе данных CAS | 103-69-5(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 7E45L4I2PS |
| Справочник по химии NIST | Benzenamine, N-ethyl-(103-69-5) |
| Система регистрации веществ EPA | N-Ethylaniline (103-69-5) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
| Коды опасности | T | |||||||||
| Заявления о рисках | 23/24/25-33 | |||||||||
| Заявления о безопасности | 28-37-45-28A | |||||||||
| РИДАДР | UN 2272 6.1/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | BX9780000 | |||||||||
| F | 8 | |||||||||
| Примечание об опасности | Toxic | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29214200 | |||||||||
| Банк данных об опасных веществах | 103-69-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats (g/kg): 0.18 i.p., 0.28 orally, 4.7 s.c. (Sziza, Podhragyai) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301+H311+H331:Токсично при проглатывании, при контакте с кожей или при вдыхании.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
P314:В случае плохого самочувствия обратиться к врачу.
N-Этиланилин химические свойства, назначение, производство
Химические свойства
N-Ethylaniline is yellow-brown oil with a weak fishy odor.Вхождение
Polyethylene bottles used in intravenous solutions have been reported to be contaminated with N-ethylaniline from rubber parts of the closure (Ulsaker and Teien 1979). It has been reported that rubber containing N,N,-dithiodimorpholine accelerator of vulcanization can release N-ethylaniline into an aqueous media (Stankevich and Shurupova 1976). This compound has also been reported as a contaminant of cigarette smoke at a level of 55.8 ng per one U.S. 85 mm cigarette (Patrianakos and Hoffmann 1979).Использование
N-Ethylaniline is used as an explosive stabilizer and in dyestuff manufacture.Методы производства
Manufacture of N-ethylaniline is based on the reaction of aniline with alkyl halide or by heating aniline with ethyl alcohol under acidic conditions followed by purification (Windholz et al 1983).Общее описание
A dark liquid with an aromatic odor. Insoluble in water. Density 0.963 g / cm3. Toxic by skin absorption and inhalation of vapors. Evolves toxic fumes during combustion. Flash point 185°F.Реакции воздуха и воды
Unstable to prolonged exposure to air and/or light. Insoluble in water.Профиль реактивности
N-Ethylaniline may react violently with nitric acid. May react with strong oxidizing agents. . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.Опасность
Toxic by ingestion, inhalation, and skin absorption.Угроза здоровью
N-Ethylaniline is considered very hazardous in a fire situation, since it is highly toxic and readily absorbed by the inhalation, dermal and oral routes (HSDB 1988). Excessive exposure causes respiratory paralysis.Пожароопасность
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.Промышленное использование
N-Ethylaniline is used as an explosives stabilizer and as an intermediate in the manufacturing of dyes and pharmaceuticals (Northcott 1978).Профиль безопасности
Poison by ingestion and intraperitoneal routes. Moderately toxic by an unspecified route. Mddly toxic by skin contact. An allergen. Flammable when exposed to heat or flame; can react with oxidizing materials. To fight fire, use dry chemical, CO2, foam. Hypergolic reaction with red fuming nitric acid. When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of aniline and NOx.Возможный контакт
This material is used as an intermediate in dyes, pharmaceuticals and explosives; in organic synthesis.Метаболизм
The metabolism of N-ethylaniline has been studied more as a tool to understanding microsomal drug metabolizing activity than as the central item of inquiry. However, the following have been clearly defined as metabolic products of N-ethylaniline: phenylhydroxylamine, N-hydroxyl, N-ethylaniline; N-ethyl-p-aminophenol; and aniline (Appel et al 1965; Heinze 1970; Hlavica 1970; Hlavica and Kiese 1969; Kampffmeyer and Kiese 1965; Kroeber et al 1970; Lange 1967 and Lange 1968). Nonmicrosomal metabolism has not been reported. Species shown capable of metabolism include rabbit, mouse, rat, dog, pig, and guinea pig with the proportions of the various metabolites often species dependent. Compounds similar to N-ethylaniline such as N-methyl-N-ethylaniline can form N-ethylaniline via demethylation (Gorrod et al 1975a,b).Перевозки
UN2272 N-Ethylaniline, Hazard Class: 6.1; Labels: 6.1-Poisonous materialsНесовместимости
May form explosive mixture with air. Decomposes on contact with light or air. Reacts with many materials. Neutralizes acids in exothermic reactions to form salts plus water. Flammable gaseous hydrogen may be generated in combination with strong reducing agents such as hydrides, nitrides, alkali metals, and sulfides. Contact with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials; strong acids, such as nitric acid, can cause fire; explosions with formation of toxic vapors of aniline and oxides of nitrogen; strong bases, isocyanates, halogenated organics, phenols (acidic), epoxides, anhydrides, and acid halidesN-Этиланилин запасные части и сырье
сырьё
запасной предмет
- [2 - [[4 - [(2-циано-4-нитрофенил) азо] фенил] этиламино] этил] триметиламмоний метилсульфат
- Acid Violet 17
- Disperse Blue 257
- [2-[[4-[(2-chloro-4-nitrophenyl)azo]phenyl]ethylamino]ethyl]trimethylammonium
- Reactive Blue BRF
- Disperse Violet 77
- Disperse Scarlet SE-R
- ЦИНК этилфенилдитиокарбамат
- Reactive Red ME-2G
- 4-(2-(N-PHENYL-N-ETHYLAMINO)ETHOXY)-3,5-DICHLOROBENZENAMINE
1of4
N-Этиланилин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-25-86736270 +86-15905172087 |
China | 103 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-25-86655873 +8613962173137 |
China | 193 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| 86-22-66880623 +8618622897568 |
China | 571 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |