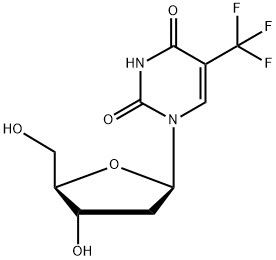
Трифлуридин
- английское имяTrifluridine
- CAS №70-00-8
- CBNumberCB6768812
- ФормулаC10H11F3N2O5
- мольный вес296.2
- EINECS200-722-8
- номер MDLMFCD00006534
- файл Mol70-00-8.mol
| Температура плавления | 190-193 °C (lit.) |
| плотность | 1.4365 (estimate) |
| показатель преломления | 50 ° (C=1, H2O) |
| температура хранения | 2-8°C |
| растворимость | Soluble in DMSO (up to 25 mg/ml) or in Water (up to 14 mg/ml) |
| форма | solid |
| пка | pKa 7.85 (Uncertain) |
| цвет | Off-white |
| Мерк | 14,9687 |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 1 month. |
| Справочник по базе данных CAS | 70-00-8(CAS DataBase Reference) |
| FDA UNII | RMW9V5RW38 |
| Словарь наркотиков NCI | trifluridine |
| Код УВД | L01BC59,S01AD02 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 20/21/22-40 | |||||||||
| Заявления о безопасности | 22-36 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | XP2087500 | |||||||||
| Примечание об опасности | Keep Cold | |||||||||
| кода HS | 29349990 | |||||||||
| Банк данных об опасных веществах | 70-00-8(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 intraperitoneal in mouse: 1931mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Трифлуридин химические свойства, назначение, производство
Описание
Trifluridine (trifluorothymidine, TFT), a fluorinated pyrimidine nucleoside, is an anti-herpesvirus agent and an antitumor antimetabolite agent. It is an analog of thymidine which inhibits thymidylate synthase possesses antiviral and anticancer activity. After phosphorylation by thymidine kinase, it is incorporated into DNA where it induces DNA-damage and interferes with repair enzymes. Enhances frame shift insertion and deletion in CRISPR genome editing in pluripotent stem cells.Химические свойства
White to Off-White SolidИспользование
Trifluridine is used as anti-herpesvirus antiviral agent in ophthalmie preparations.Определение
ChEBI: Trifluridine is a pyrimidine 2'-deoxyribonucleoside compound having 5-trifluoromethyluracil as the nucleobase. An antiviral drug used mainly in the treatment of primary keratoconjunctivitis and recurrent epithelial keratitis. It has a role as an antiviral drug, an antimetabolite, an EC 2.1.1.45 (thymidylate synthase) inhibitor and an antineoplastic agent. It is a nucleoside analogue, an organofluorine compound and a pyrimidine 2'-deoxyribonucleoside.Показания
Trifluridine (Viroptic) is a fluorinated pyrimidine nucleoside that has in vitro activity against HSV-1 and HSV- 2, vaccinia, and to a lesser extent, some adenoviruses. Activation of trifluridine requires its conversion to the 5 monophosphate form by cellular enzymes.Trifluridine monophosphate inhibits the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) by thymidylate synthetase. In addition, it competes with deoxythymidine triphosphate (dTTP) for incorporation by both viral and cellular DNA polymerases. Trifluridine-resistant mutants have been found to have alterations in thymidylate synthetase specificity.Общее описание
Trifluridine, 5-trifluoromethyl-29-deoxyuridine (Viroptic),is a fluorinated pyrimidine nucleoside that demonstrates invitro inhibitory activity against HSV-1 and HSV-2, CMV,vaccinia, and some adenoviruses.Trifluridine possesses atrifluoromethyl group instead of an iodine atom at the 5-position of the pyrimidine ring.synthetase, and the biologically generated triphosphatecompetitively inhibits thymidine triphosphate incorporationinto DNA by DNA polymerase. In addition, trifluridine inits triphosphate form is incorporated into viral and cellularDNA, creating fragile, poorly functioning DNA.Trifluridine is approved in the United States for the treatmentof primary keratoconjunctivitis and recurrent epithelialkeratitis caused by HSV types 1 and 2. Topical trifluridineshows some efficacy in patients with acyclovir-resistantHSV cutaneous infections.
Механизм действия
Trifluorothymidine is a fluorinated pyridine nucleoside structurally related to idoxuridine. It has been approved by the U.S. FDA and is a potent, specific inhibitor of replication of HSV-1 in vitro. Its mechanism of action is similar to that of idoxuridine. Like other antiherpes drugs, it is first phos-phorylated by thymidine kinase to mono-, di-, and triphosphate forms, which are then incorporated into viral DNA in place of thymidine to stop the formation of late virus mRNA and subsequent synthesis of the virion proteins.Фармакокине?тика
Trifluorothymidine is a synthetic halogenated pyrimidine nucleoside, first synthesized as an antitumor agent. It inhibits enzymes of the DNA pathway and is incorporated into both cellular and progeny viral DNA, causing faulty transcription of late messenger RNA and the production of incompetent virion protein. It does not require a viral thymidine kinase for monophosphorylation and is far less selective and more toxic than other analogs. It is active against HSV-1 and HSV-2, vaccinia virus, CMV and possibly adenovirus. Trifluorothymidine, when given IV, shows a plasma half-life of 18 minutes and is excreted in the urine either unchanged or as the inactive metabolite 5-carboxyuracil. When applied as a 1% ophthalmic solution, it rapidly enters the aqueous humor of HSV-infected rabbits’ eyes but is cleared within 60–90 min.Побочные эффекты
The most frequent adverse reactions to trifluridine administration are transient burning or stinging and palpebral edema. Other adverse reactions include superficial punctate keratopathy, epithelial keratopathy, hypersensitivity, stromal edema, irritation, keratitis sicca, hyperemia, and increased intraocular pressure.Trifluridine is mutagenic in vitro and carcinogenic and teratogenic when administered subcutaneously to animals. Topical trifluridine was not teratogenic in animal studies. Because it is applied topically in humans, the likelihood of systemic effects is low.
Трифлуридин запасные части и сырье
Трифлуридин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 010-62664360 +8613328773880 |
China | 117 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21670 | 55 | ||
| 008657128800458; +8615858145714 |
China | 9337 | 55 | ||
| +undefined-21-51877795 | China | 32760 | 60 | ||
| 15950718863 | CHINA | 295 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29897 | 58 | ||
| +86 18953170293 | China | 2931 | 58 | ||
| 0086-13720134139 | CHINA | 967 | 58 | ||
| +86-755-89396905 +86-15013857715 |
China | 10318 | 58 | ||
| +8615866703830 | China | 7353 | 58 |