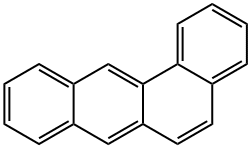
1,2-бензантрацен
- английское имя1,2-Benzanthracene
- CAS №56-55-3
- CBNumberCB6690674
- ФормулаC18H12
- мольный вес228.29
- EINECS200-280-6
- номер MDLMFCD00003599
- файл Mol56-55-3.mol
| Температура плавления | 157-159 °C(lit.) |
| Температура кипения | 437.6 °C(lit.) |
| плотность | 1.274 g/cm3 |
| давление пара | 54.8 (de Kruif, 1980) |
| показатель преломления | 1.7710 (estimate) |
| Fp | -18 °C |
| температура хранения | 2-8°C |
| растворимость | Soluble in ethanol, ether, acetone, benzene (U.S. EPA, 1985), toluene, xylenes, and other monoaromatic hydrocarbons. |
| пка | >15 (Christensen et al., 1975) |
| форма | Solid |
| цвет | Light Yellow |
| Растворимость в воде | 9.4ug/L(25 ºC) |
| Мерк | 14,1062 |
| БРН | 1909298 |
| констант закона Генри | 1.48, 3.06, 6.22, 12.0, and 20.8 at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al., 1998) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Справочник по базе данных CAS | 56-55-3(CAS DataBase Reference) |
| FDA UNII | C5PLF6152K |
| Предложение 65 Список | Benz(a)anthracene |
| МАИР | 2B (Vol. 92, Sup 7) 2010 |
| Система регистрации веществ EPA | Benz[a]anthracene (56-55-3) |
| UNSPSC Code | 77101502 |
| NACRES | NA.24 |
| Коды опасности | T,N,Xn,F | |||||||||
| Заявления о рисках | 45-50/53-67-65-38-11-63-43-36/37/38-23/24/25-39/23/24/25-52/53-40-51/53-20 | |||||||||
| Заявления о безопасности | 53-45-60-61-62-36/37-24/25-23-26-33-25-16-9 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | CV9275000 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29029000 | |||||||||
| Банк данных об опасных веществах | 56-55-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 for mice by intravenous injection 10 mg/kg (Patnaik, 1992). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
1,2-бензантрацен химические свойства, назначение, производство
Описание
1,2-Benzanthracene is available as colourless to yellow-brown fluorescent flakes or powder. It is stable, combustible, and incompatible with strong oxidising agents. On decomposition, 1,2-benzanthracene releases carbon monoxide, carbon dioxide, acrid smoke, and fumes. Exposures may cause irritation of the eyes, skin, and respiratory tract.Химические свойства
1,2-Benzanthracene is available as colorless to yellow brown fl uorescent fl akes or powder. It is stable, combustible, and incompatible with strong oxidizing agents. On decomposition, 1,2-benzanthracene releases carbon monoxide, carbon dioxide, acrid smoke, and fumes. During work, 1,2-benzanthracene can be absorbed into the body of occupational workers by inhalation, through the skin, and by ingestion. Exposures may cause irritation to the eyes, skin, and respiratory tract.Физические свойства
Colorless leaflets or plates with a greenish-yellow fluorescenceИспользование
Benz[a]anthracene is a PAH that has carcinogenic properties. It is also used in the synthesis of anti-tumor agents.Общее описание
Colorless leaflets or plates or coarse gold powder with a greenish-yellow fluorescence. May reasonably be expected to be a carcinogen.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
1,2-BENZANTHRACENE may react vigorously with strong oxidizing agents. Can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction.Опасность
Confirmed carcinogen. Found in oils, waxes, smoke, food, drugs.Угроза здоровью
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1,2-BENZANTHRACENE emits acrid smoke and irritating fumes.Пожароопасность
Flash point data for 1,2-BENZANTHRACENE are not available. 1,2-BENZANTHRACENE is probably combustible.Профиль безопасности
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data by skin contact and other routes. Poison by intravenous route. Human mutation data reported. It is found in oils, waxes, smoke, food, drugs. When heated to decomposition it emits acrid smoke and irritating fumes.Канцерогенность
BA’s metabolites are genotoxic in the Ames mutation test and caused unscheduled DNA synthesis in primary rat hepatocytes.In an in vivo mutagenic assay, male CD rats (6/group) were dosed three times with BA over a 24-hour interval by intratracheal instillation. Lung cells were enzymatically separated and used to determine the frequency of DNA adducts, sister chromatid exchanges (SCEs), and micronuclei. BA induced DNA adducts, SCEs, and micronuclei in this rat lung cell system.Benz(a)anthracene is designated an A2- suspected human carcinogen by ACGIH and has no assigned threshold limit value.
хранилище
Store in a cool, dry, well-ventilated area away from incompatible substances. Keep containers tightly closedПеревозки
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.Методы очистки
Crystallise 1,2-benzanthracene from MeOH, EtOH or *benzene (charcoal), then chromatograph it on alumina from sodium-dried *benzene (twice), using vacuum distillation to remove *benzene. Final purification is by vacuum sublimation. [Beilstein 5 IV 2549.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Powder can form an explosive mixture with air.Утилизация отходов
Atomize into incinerator with a flammable liquid.Меры предосторожности
Workers should wash thoroughly after using and handling 1,2-benzanthracene. Use only in a well-ventilated area. Minimize dust generation and accumulation. Avoid contact with the eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.1,2-бензантрацен запасные части и сырье
1,2-бензантрацен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618903931531 | China | 179 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-755-23284190 13684996853 | CHINA | 304 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | ||
| 18017061086 | CHINA | 5600 | 58 | ||
| 571-88938639 +8617705817739 |
China | 52849 | 58 | ||
| +8617653113219 | China | 2737 | 58 | ||
| +49-7246-3082843 | Germany | 1859 | 58 | ||
| +8619901505185 | China | 528 | 58 |