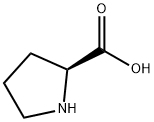
L-Пролин
- английское имяL-Proline
- CAS №147-85-3
- CBNumberCB6118196
- ФормулаC5H9NO2
- мольный вес115.13
- EINECS205-702-2
- номер MDLMFCD00064318
- файл Mol147-85-3.mol
химическое свойство
| Температура плавления | 228 °C (dec.) (lit.) |
| Температура кипения | 215.41°C (rough estimate) |
| альфа | -85.5 º (c=4, H2O) |
| плотность | 1.35 |
| Плотность накопления | 500kg/m3 |
| давление пара | 0Pa at 25℃ |
| FEMA | 3319 | L-PROLINE |
| показатель преломления | -85 ° (C=4, H2O) |
| температура хранения | 2-8°C |
| растворимость | H2O: 50 mg/mL |
| форма | powder |
| пка | 1.95, 10.64(at 25℃) |
| цвет | White |
| РН | 6.0-7.0 (25℃, 1M in H2O) |
| Запах | at 100.00 %. odorless |
| Odor Type | odorless |
| Биологические источники | synthetic |
| оптическая активность | [α]20/D 85.0±1.0°, c = 5% in H2O |
| Растворимость в воде | soluble |
| Чувствительный | Hygroscopic |
| λмакс | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| Номер JECFA | 1425 |
| Мерк | 14,7780 |
| БРН | 80810 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | ONIBWKKTOPOVIA-UHFFFAOYSA-N |
| LogP | -2.54 at 20℃ |
| Вещества, добавляемые в пищу (ранее EAFUS) | L-PROLINE |
| FDA 21 CFR | 172.320 |
| Справочник по базе данных CAS | 147-85-3(CAS DataBase Reference) |
| FDA UNII | 9DLQ4CIU6V |
| Справочник по химии NIST | Proline(147-85-3) |
| Система регистрации веществ EPA | L-Proline (147-85-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.26 |
больше
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38-22 | |||||||||
| Заявления о безопасности | 24/25-36/37/39-26 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | TW3584000 | |||||||||
| F | 3-10 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29339990 | |||||||||
| Банк данных об опасных веществах | 147-85-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 5110 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
L-Пролин химические свойства, назначение, производство
Химические свойства
L-Proline, an amino acid, is colorless to white crystal or crystalline powder that has a slight, characteristic odor with a slightly sweet taste. It is soluble in water, insoluble in ethanol, diethyl ether and n-butanol, yellow in case of hydrated ninhydrin test solution, glacial acetic acid Red after acidification; pH=6.3, decomposition point is 220-222°C; specific optical rotation [α]20D-85° (0.5-2.0mg/ml, H2O), [α]20D-60.4° (0.5-2.0mg /ml, 5mol/LHCl). It is synthesized from L-glutamine and L-glutamate via L-ornithine in intestine, and from L-ornithine in liver. It is widely used as an ingredient in infusion and infant formula.Вхождение
Reported found as a component in many proteins; also widely occurring as the free acid in natural products. A major constituent of collagen, the main fibrous protein found in bone, cartilage and other connective tissue.Использование
L-Proline is an amino acid and precursor (with vitamin C) for collagen, the building block of the structure of tendons, ligaments, arteries, veins and muscles. It is important in wound healing.Подготовка
Synthesis of L-proline: Using glutamic acid as a raw material, it is esterified with absolute ethanol under the catalysis of sulfuric acid, and triethanolamine is added to free the aminosulfate to obtain glutamic acid-δ-ethyl ester. The glutamic acid-δ-ethyl ester is then reduced with a metal reducing agent potassium borohydride to obtain crude proline, which is finally separated and purified to obtain crude L-proline.Определение
ChEBI: L-proline is pyrrolidine in which the pro-S hydrogen at position 2 is substituted by a carboxylic acid group. L-Proline is the only one of the twenty DNA-encoded amino acids which has a secondary amino group alpha to the carboxyl group. It is an essential component of collagen and is important for proper functioning of joints and tendons. It also helps maintain and strengthen heart muscles. It has a role as a micronutrient, a nutraceutical, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a member of compatible osmolytes. It is a glutamine family amino acid, a proteinogenic amino acid, a proline and a L-alpha-amino acid. It is a conjugate base of a L-prolinium. It is a conjugate acid of a L-prolinate. It is an enantiomer of a D-proline. It is a tautomer of a L-proline zwitterion.Биотехнологическое производство
Direct fermentation using analogue-resistant mutants of coryneform bacteria or Serratia marcescens is an economic production method. An isoleucine auxotrophic mutant of Brevibacterium flavum having resistance to sulfaguanidine and D,L-3,4-dehydroproline (DP) is able to accumulate 40 g/L L-proline. Brevibacterium flavum AP113 is claimed to produce 97.5 g/L L-proline; this mutant is characterized by isoleucine auxotrophy, resistance to DP, and osmotic pressure and incapable to degrade Lproline. A proline oxidase-less strain of Serratia marcescens, having resistance to DP, thiazoline-4-carboxylate and azetidine-2-carboxylate, overproduces 58.5 g/L L-proline into the culture medium. By amplification of the genes proA and proB in this type of regulatory mutant, a construct was obtained which yields 75 g/L L-proline.преимущество
L-proline is considered a non-essential amino acid as it can be synthesised from arginine via the urea cycle in liver, and from glutamine/glutamic acid in the intestinal epithelium. It has a number of beneficial properties including connective tissue strengthening, Stronger Connective Tissue, Decreased Risk Of Heart Disease, Maintenance Of Muscle Tissueand skin health.Общее описание
L-Proline is a non-essential amino acid, which is a building block of proteins. Peptides bond to proline, making it a useful building block for proteins. It can be used as a cell culture media component for the commercial biomanufacturing of therapeutic recombinant proteins and monoclonal antibodies. L-Proline plays important roles in various biological processes. It is involved in the synthesis of collagen, which is one of the most abundant proteins in the human body and provides structural support to tissues such as skin, bone, cartilage, and tendons.Побочные эффекты
The only known side effects are reactions from taking too much L-proline, like all amino acids. It causes toxicity levels and amino acid imbalances in your body.Методы очистки
A likely impurity is hydroxyproline. Purify L-proline via its picrate which is crystallised twice from water, then decomposed with 40% H2SO4. The picric acid is extracted with diethyl ether, the H2SO4 in solution is precipitated with Ba(OH)2, and the filtrate is evaporated. The residue is crystallised from hot absolute EtOH [Mellan & Hoover J Am Chem Soc 73 3879 1951] or EtOH/Et2O. Its solubility in H2O is >100%. It sublimes at 182-187o/0.3mm with 99.4% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. It is hygroscopic and is stored in a desiccator. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2178-2199 1961, Beilstein 22 III/IV 8, 22/1 V 31.]L-Пролин запасные части и сырье
сырьё
1of3
запасной предмет
- D-(S)-3-acetylthio-2-methylpropionylL-proline
- R(-)-ДЕНОПАМИН
- Эналаприл
- D-пролин
- (S)-(-)-2-метил-CBS-оксазаборoлидин
- DL-пролин
- Levosulpiride
- 2-Фтор-4- (метилсульфонил) анилин
- (S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol
- Лизиноприл
1of4
L-Пролин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86 18953170293 | China | 2930 | 58 | ||
| +86-0555-8889890 +86-17356584060 |
China | 72 | 58 | ||
| +86-15800805830 +86-15800805830 |
China | 184 | 58 | ||
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 |