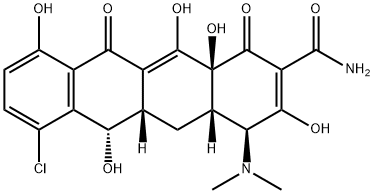
Демеклоциклин
- английское имяdemeclocycline
- CAS №127-33-3
- CBNumberCB5918843
- ФормулаC21H21ClN2O8
- мольный вес464.85
- EINECS204-834-8
- номер MDLMFCD00864922
- файл Mol127-33-3.mol
химическое свойство
| Температура плавления | 176°C (rough estimate) |
| Температура кипения | 787.1±60.0 °C(Predicted) |
| плотность | 1.3118 (rough estimate) |
| показатель преломления | 1.6000 (estimate) |
| растворимость | H2O:1.5(Max Conc. mg/mL);3.23(Max Conc. mM) |
| форма | Solid |
| пка | 4.50±1.00(Predicted) |
| цвет | Green to dark green |
| Растворимость в воде | 1.4g/L(25 ºC) |
| FDA UNII | 5R5W9ICI6O |
| Банк данных об опасных веществах | 127-33-3(Hazardous Substances Data) |
Демеклоциклин химические свойства, назначение, производство
Описание
Demethylchlortetracycline was isolated from the culture broth of a mutant of Streptomyces aureofaciens, the chlortetracycline-producing strain, by Lederle Research Laboratories in 1957. It shows one and one-half to two times as much in vitro antimicrobial activity and in vivo protective effect as tetracycline. Its base and hydrochloride have been used orally and by topical application to treat infections caused by Staphylococcus, Streptococcus, Rickettsia, Chlamydiae, Neisseria, Klebsiella, Proteus, Escherichia coli, and Haemophilus influenzae.Использование
Demeclocycline, a chlortetracycline analogue produced by a mutagenised strain of Streptomyces aureofaciens, was first isolated in 1957. Like all tetracyclines, demeclocycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal subunits, blocking protein synthesis. Demeclocycline has been extensively cited in the literature with over 800 references relating almost exclusively to in vivo use.Определение
ChEBI: Tetracycline which lacks the methyl substituent at position 7 and in which the hydrogen para- to the phenolic hydroxy group is substituted by chlorine. Like tetracycline, it is an antibiotic, but being excreted more slowly, effective blood lev ls are maintained for longer. It is used (mainly as the hydrochloride) for the treatment of Lyme disease, acne and bronchitis, as well as for hyponatraemia (low blood sodium concentration) due to the syndrome of inappropriate antidiuretic hormone (SIADH) w ere fluid restriction alone has been ineffective.Профиль безопасности
Poison by intravenous and intraperitoneal routes. Human systemic effects by ingestion: diabetes insipidus, urine volume increase, other changes in urine composition, dermatitis, changes in the nails, allergic rhinitis, serum sickness, effects on cyclic nucleotides. Human reproductive effects by an unspecified route: postnatal measures or effects on newborn. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits very toxic fumes of Cland NOx.Демеклоциклин запасные части и сырье
сырьё
Демеклоциклин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86 18953170293 | China | 2930 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| United States | 38631 | 58 | ||
| +8618058761490 | China | 49983 | 58 | |
| +8618327326525 | China | 8467 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +86-16631818819 +86-17736933208 |
China | 9300 | 58 |