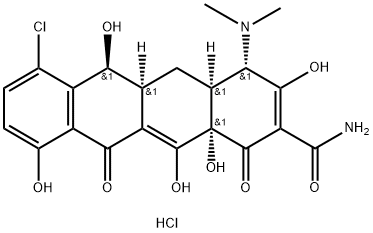
Demeclocycline гидрохлорид
- английское имяDemeclocycline hydrochloride
- CAS №64-73-3
- CBNumberCB3272226
- ФормулаC21H22Cl2N2O8
- мольный вес501.31
- EINECS200-592-2
- номер MDLMFCD00864922
- файл Mol64-73-3.mol
| Температура плавления | >245°C (dec.) |
| температура хранения | Sealed in dry,Store in freezer, under -20°C |
| растворимость | H2O: 20 mg/mL, clear, very deep greenish yellow |
| форма | powder |
| пка | pKa 3.3 (Uncertain) |
| цвет | Light Yellow to Yellow |
| Растворимость в воде | Soluble in water. |
| Мерк | 13,2905 |
| БРН | 5705221 |
| ИнЧИКей | GVSJQNRGSCOSNJ-KBHRXELFSA-N |
| Справочник по базе данных CAS | 64-73-3(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | Declomycin; demeclocycline hydrochloride |
| FDA UNII | 29O079NTYT |
| Словарь наркотиков NCI | Declomycin |
| Код УВД | D06AA01,J01AA01 |
| Предложение 65 Список | Demeclocycline Hydrochloride (internal use) |
| Система регистрации веществ EPA | 2-Naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-1,11-dioxo-, hydrochloride (1:1), (4S,4aS,5aS,6S,12aS)- (64-73-3) |
| UNSPSC Code | 12352207 |
| NACRES | NA.75 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 43 | |||||||||
| Заявления о безопасности | 36/37 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | QI7700000 | |||||||||
| F | 8-10-21 | |||||||||
| кода HS | 29413020 | |||||||||
| Токсичность | LD50 orally in rats: 2372 mg/kg (Goldenthal) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Demeclocycline гидрохлорид химические свойства, назначение, производство
Описание
Demeclocycline lacks the C-6-methyl of tetracycline and is produced by a genetically altered strain of Streptomyces aureofaciens. Because it is a secondary alcohol, it is more chemically stable than tetracycline against dehydration. Food and milk co-consumption decrease absorption by half, although it is 60 to 80% absorbed by fasting adults. It is the tetracycline most highly associated with phototoxicity and has been shown to produce dose-dependent, reversible diabetes insipidus with extended use.Химические свойства
Yellow SolidИспользование
Demeclocycline hydrochloride is a salt prepared from demeclocycline taking advantage of the basic dimethylamino group which protonates and readily forms a salt in hydrochloric acid solutions. The hydrochloride is the preferred formulation for pharmaceutical applications. Like all tetracyclines, demeclocycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal subunits, blocking protein synthesis.Определение
ChEBI: The hydrochloride salt of demeclocycline. A tetracycline antibiotic, it is used (mainly as the hydrochloride) for the treatment of Lyme disease, acne and bronchitis, as well as for hyponatraemia (low blood sodium concentration) due to the syndrome of inapp opriate antidiuretic hormone (SIADH) where fluid restriction alone has been ineffective.Антимикробная активность
Occasional strains of viridans streptococci, N. gonorrhoeae and H. influenzae are more susceptible than to tetracycline. It is the most active tetracycline against Brucella spp.Общее описание
Demeclocycline, 7-chloro-6-demethyltetracycline (Declomycin),was isolated in 1957 by McCormick et al. from amutant strain of S. aureofaciens. Chemically, it is 7-chloro-4-(dimethylamino)1,4,4a,5,5a,6, 11, 12a-octahydro-3, 6, 10, 12,12a-pentahydroxy1, 11-dioxo2-naphthacenecarboxamide.Thus, it differs from chlortetracycline only in the absence ofthe methyl group on C-6.Demeclocycline is a yellow, crystalline powder that isodorless and bitter. It is sparingly soluble in water. A 1% solutionhas a pH of about 4.8. It has an antibiotic spectrumlike that of other tetracyclines, but it is slightly more activethan the others against most of the microorganisms forwhich they are used. This, together with its slower rate ofelimination through the kidneys, makes demeclocycline aseffective as the other tetracyclines, at about three fifths ofthe dose. Like the other tetracyclines, it may cause infrequentphotosensitivity reactions that produce erythema afterexposure to sunlight. Demeclocycline may produce this reactionsomewhat more frequently than the other tetracyclines.The incidence of discoloration and mottling of theteeth in youths from demeclocycline appears to be as low asthat from other tetracyclines.
Фармацевтические приложения
6-Demethyl-7-chlortetracycline. A fermentation product of a mutant strain of Streptomyces aureofaciens formulated as the hydrochloride for oral administration.Фармакокине?тика
Oral absorption: 60–70%Cmax 300 mg oral:2 mg/L after 3–6 h
Plasma half-life:c.12 h
Volume of distribution:c.1.7 L/kg
Plasma protein binding:90%
Absorption
It is promptly yet incompletely absorbed by mouth, giving mean peak plasma levels after a single dose that are slightly higher than those produced by oxytetracycline and chlortetracycline, but lower than those achieved by tetracycline. However, with repeat dosing, steady-state concentrations exceed those for tetracycline. Simultaneous administration of antacids markedly depresses blood levels.
Distribution and excretion
It is widely distributed, achieving concentrations in pleural exudates
similar to those of blood. CSF penetration is poor, especially in the absence of inflammation. Biliary concentrations
are 20–30 times higher than those of plasma, and 40–50% of the drug can be recovered from feces. The other route of elimination is via glomerular filtration without reabsorption and accumulation occurs in renal failure.
Клиническое использование
It has been extensively used in the management of the syndrome of inappropriate ADH secretion in a dose of at least 1.2 g per day; therapeutic response may take several days, but is superior to that of lithium. It has also found occasional use in patients with water retention as a result of congestive cardiac failure and in those with alcoholic cirrhosis and water and electrolyte retention.Побочные эффекты
Untoward reactions, notably gastrointestinal intolerance, are generally those typical of the group. Occasional patients develop transient steatorrhea.Of particular note is the occurrence of nephrogenic diabetes insipidus with development of vasopressin-resistant polyuria. The effect is dose dependent and occurs with daily doses in excess of 1.2 g. The drug inhibits activation of adenylate cyclase and protein kinase, which are both important in the interaction of antidiuretic hormone (ADH) with receptors within the renal tubule, thus decreasing the effect of ADH on the kidney. As a result, it has found a place in the treatment of inappropriate ADH secretion.
Renal failure may occur, particularly if prescribed for those with advanced liver cirrhosis. The mechanism is uncertain but may in part be related to the antianabolic effect of the tetracyclines as well as a direct toxic effect.
Photosensitivity may be severe and accompanied by vesiculation, edema and onycholysis. It is largely restricted to exposed skin; patients should avoid prolonged exposure to sunlight.
Методы очистки
Crystallise the salt from EtOH/Et2O or H2O and dry it in air [McCormick et al. J Am Chem Soc 79 4561 1957, Dobrynin et al. Tetrahedron Lett 901 1962]. [Beilstein 14 IV 2625.]Demeclocycline гидрохлорид запасные части и сырье
Demeclocycline гидрохлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0571-28186870; +undefined8613073685410 |
China | 1015 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| 025-83697070 | CHINA | 3009 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 027-85778276 | CHINA | 176 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 |