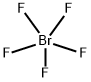
ПЕНТАФТОРИД БРОМА
- английское имяBromine pentafluoride
- CAS №7789-30-2
- CBNumberCB5301973
- ФормулаBrF5
- мольный вес174.9
- EINECS232-157-8
- номер MDLMFCD00042564
- файл Mol7789-30-2.mol
химическое свойство
| Температура плавления | -62,5°C |
| Температура кипения | 40,3°C |
| плотность | 2,48 g/cm3 |
| растворимость | reacts with H2O (explodes, explosive) |
| форма | Pale yellow liquid at temperatures below 40.3°C; pungent, corrosive gas at temperatures above 40.3°C. |
| цвет | colorless |
| Растворимость в воде | Insoluble |
| Пределы воздействия | TLV-TWA 0.1 ppm (~0.7 mg/m3) (ACGIH and MSHA), 2.5 mg(F)/m3 (OSHA). |
| Стабильность | Strong oxidizing agent. Reacts with water, combustible materials, acids, metals. Explosion risk. |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | UPI6B7Y9UQ |
| Справочник по химии NIST | Bromine pentafluoride(7789-30-2) |
| Система регистрации веществ EPA | Bromine pentafluoride (7789-30-2) |
| Коды опасности | O | |||||||||
| Заявления о рисках | 8-23/24/25-34 | |||||||||
| Заявления о безопасности | 7/8-36/37/39-45 | |||||||||
| РИДАДР | 1745 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.1 ppm (0.7 mg/m3) | |||||||||
| Примечание об опасности | Oxidising agent | |||||||||
| Класс опасности | 5.1 | |||||||||
| Группа упаковки | I | |||||||||
| Банк данных об опасных веществах | 7789-30-2(Hazardous Substances Data) | |||||||||
| ИДЛА | 1.7 ppm | |||||||||
| NFPA 704: |
|
ПЕНТАФТОРИД БРОМА химические свойства, назначение, производство
Химические свойства
Bromine pentafluoride is colorless to pale yellow fuming liquid, with pungent odor. At temperatures above boiling point this chemical is a colorless gas.Физические свойства
Colorless to pale yellow liquid; fumes in air; density 2.466 g/mL at 25°C; boils at 40.8°C; decomposes above 460°C; solidifies at -60.5°C; reacts violently with water.Использование
Bromine pentafluoride (BrF5) is very corrosive to the skin and explodes when in contact with water. It is used as an oxidizer in rocket fuel.Подготовка
Bromine pentafluoride is prepared by fluorination of bromine at 200°C. The reaction is carried out in an iron or copper vessel. The halogens are diluted in nitrogen.Общее описание
A colorless, fuming liquid with a pungent odor. Used to make other chemicals and in rockets. Very toxic by inhalation. Corrosive to metals and tissue. Will accelerate the burning of combustible material. If the containers are involved in a fire they may rupture violently and rocket.Реакции воздуха и воды
Produces corrosive fumes in moist air. Reacts explosively upon contact with water. Very reactive, usually with conflagration [Merck, 11th ed. 1989]. Reaction with water is violent, oxygen being evolved, [Mellor 2 Supp. 1:172 1956].Профиль реактивности
Bromine pentafluoride an oxidizing agent. Is decomposed exothermically by water to hydrofluoric acid and other materials. Reacts with these other hydrogen-containing substances (among others) vigorously enough to cause a fire or explosion: acetic acid, ammonia, benzene, ethanol, hydrogen, hydrogen sulfide, methane, cork, grease paper, wax. Mixtures with acids, halogens, metal halides, metals, nonmetals, or metal oxides at ambient or slightly above ambient temperatures have resulted in violent reaction (nitric acid, sulfuric acid, chlorine, iodine, ammonium chloride, potassium iodide, boron powder, selenium, tellurium, aluminum powder, bismuth, cobalt powder, iron powder, arsenic, nickel powder, chromium trioxide, charcoal, red phosphorus, sulfur dioxide, magnesium oxide. Solutions of acetonitrile and 9% Bromine pentafluoride have been found to decompose violently at ambient temperatures. Mixtures of perchloryl perchlorate and Bromine pentafluoride form shock sensitive explosives. [Bretherick, 5th ed., 1995, p. 640].Опасность
Corrosive to skin and tissue. Explodes on contact with water. Eye and upper respiratory tract irritant.Угроза здоровью
Bromine pentafluoride is more active andtoxic than elemental fluorine or bromine tri-fluoride. The liquid is severely corrosive tothe skin. The vapors are highly irritatingto the eyes, skin, and mucous membranes.Exposure to 500 ppm vapor caused gasp ing, swelling of eyelids, cloudiness of thecornea, lacrimation, salivation, and respira tory distress in test animals (ACGIH 1986).A few minutes’ exposure to 100 ppm waslethal to most experimental animals. Chronicexposure can cause nephrosis and hepatosis.Ingestion of a few drops can cause severecorrosion and burn the mouth.Пожароопасность
Noncombustible liquid; stable to heat, shock, and electric spark. However, bromine penta- fluoride is a highly reactive substance. It reacts explosively with water, producing toxic and corrosive fumes. It decomposes in contact with acids, producing toxic fumes of bromine and fluorine. The reaction is violent, especially with concentrated nitric or sulfuric acid.Bromine pentafluoride reacts violently with organic materials, such as carboxylic acids, alcohols, ethers, hydrocarbons, grease,wax, and cellulose. Spontaneous flaming and/or explosion can occur when mixed with these or any other organic compound. Reac tions with metals in powder form and/or upon warming can result in a violent explo sion. Ignition occurs under cold condition. Violent reactions occur with metal halides, oxides, and sulfides. Bromide pentafluo ride ignites when mixed with iodine and explodes with chlorine on heating. Mixtures of bromine pentafluoride with sulfur, phos phorus, and carbon ignite.
Avoid using water in the case of fire, since the compound explodes with water. How ever, water may be used from a safe distance to extinguish a fire involving large amounts of combustible materials, and to keep the fire- exposed containers cool. Fires involving small amounts of combustible material may be extinguished by CO2 or a dry chemical (NFPA 1997).
Профиль безопасности
A poisonous, corrosive, and extremely reactive gas. It is a powerful oxidizer. Will react with water or steam to produce toxic and corrosive fumes. The liquefied gas reacts violently with many organic compounds and some inorganic compounds. Explodes or ignites on contact with hydrogen-containing materials (e.g., acetic acid, ammonia, benzene, ethanol, hydrogen, hydrogen sulfide, methane, cork, grease, paper, wax, chloromethane). Reacts violently and may i p t e on contact with acids, halogens, nonmetals, metal hahdes, metals, oxides, concentrated nitric or sulfuric acids, aluminum powder, ammonium chloride, antimony, arsenic, arsenic pentoxide, barium, bismuth, boron powder, boron trioxide, calcium oxide, carbon monoxide, charcoal, chlorine, chromium, chromium trioxide, cobalt powder, iodine, iodne pentoxide, iridium powder, iron powder, lithium powder, manganese, magnesium oxide, molybdenum, molybdenum trioxide, nickel powder, red phosphorus, phosphorus pentoxide, selenium, sulfur, sulfur dioxide, tellurium, tungsten, tungsten trioxide, water, zinc. When heated to decomposition it emits very toxic fumes of Fand Br-. See also BROMINE and FLUORIDES.Возможный контакт
Bromine pentafluoride is used as an oxidizer in liquid rocket propellant combinations; it may be used in chemical synthesis.Перевозки
UN1745 Bromine pentafluoride 5.1; Labels: 5.1—Oxidizer, 6.1—Poison Inhalation Hazard, 8— Corrosive material, Inhalation Hazard Zone A.Методы очистки
Purify it via its KF complex, as described for chlorine trifluoride. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 158-159 1963.] HIGHLY TOXIC.Несовместимости
A powerful oxidizer. Bromine pentafluoride reacts with every known element except inert gases, nitrogen, and oxygen. It reacts violently with water, acids, acid fumes (releasing highly toxic fumes of bromine and fluorine). Incompatible with halogens, arsenic, selenium, alkaline halides, sulfur, iodine, glass, metallic halides, metal oxides, and metals (except copper, stainless steel; nickel, and Monel). Fire may result from contact with combustibles or organic matter at room temperature, and contact of this substance with water produces an explosion. Even under mild conditions this substance attacks organic compounds vigorously, often causing explosion. Decomposes in heat above 460° C.Утилизация отходов
Allow gas to flow into mixed caustic soda and slaked lime solution. Return unwanted cylinders to supplier if possible.ПЕНТАФТОРИД БРОМА поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 021-61259108 18621169109 |
China | 40228 | 62 | |
| 0510-051082803581 15903302207 |
China | 229 | 58 | |
| 159-0619-7626 4001882517 |
China | 528 | 58 | |
| 18062587608 | China | 7665 | 58 |