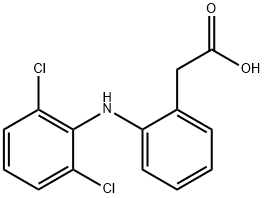
1-(2,6-Dichlorophenyl)-2-indolinone
- русский язык имя
- английское имя1-(2,6-Dichlorophenyl)-2-indolinone
- CAS №15307-86-5
- CBNumberCB5225367
- ФормулаC14H11Cl2NO2
- мольный вес296.15
- EINECS239-348-5
- номер MDLMFCD00056694
- файл Mol15307-86-5.mol
| Температура плавления | 156-158° |
| Температура кипения | 412.0±45.0 °C(Predicted) |
| плотность | 1.431±0.06 g/cm3(Predicted) |
| температура хранения | Sealed in dry,Room Temperature |
| растворимость | soluble in Methanol |
| форма | powder to crystal |
| пка | pKa 4 (Uncertain) |
| цвет | White to Almost white |
| Растворимость в воде | 1.278mg/L(30 ºC) |
| Мерк | 14,3081 |
| ИнЧИКей | DCOPUUMXTXDBNB-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 15307-86-5(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | diclofenac |
| FDA UNII | 144O8QL0L1 |
| Код УВД | D11AX18,M01AB05,M01AB55,M02AA15,S01BC03 |
| Справочник по химии NIST | Benzeneacetic acid, 2-[(2,6-dichlorophenyl)amino]-(15307-86-5) |
| Система регистрации веществ EPA | Benzeneacetic acid, 2-[(2,6-dichlorophenyl)amino]- (15307-86-5) |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
| РИДАДР | 3249 | |||||||||
| RTECS | AG6310000 | |||||||||
| кода HS | 2922.49.2600 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 15307-86-5(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H411:Токсично для водных организмов с долгосрочными последствиями.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
1-(2,6-Dichlorophenyl)-2-indolinone химические свойства, назначение, производство
Использование
prostaglandin synthetic inhibitorПоказания
Diclofenac (Voltaren, Cataflam) is approved for use in rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, dysmenorrhea, and topically for the treatment treatment of ocular inflammation and actinic keratosis. Diclofenac exhibits approximately equal selectivity for COX-1 and COX-2. The most common adverse reactions are GI disturbances and headache.A reversible elevation of serum transaminases occurs in 15% of patients.Биологические функции
Diclofenac (Voltaren) is a phenylacetic acid derivative that is a potent inhibitor of COX and that has analgesic, antiinflammatory, and antipyretic effects. Its use is accompanied by side effects similar to those of other NSAIDs. Indications for the drug include rheumatoid arthritis, osteoarthritis, and ophthalmic inflammation (use of an ophthalmic preparation).Механизм действия
Diclofenac is unique among the NSAIDs in that it possesses three possible mechanisms of action: 1) inhibition of the arachidonic acid cyclooxygenase system (3 to 1,000 times more potent than other NSAIDs on a molar basis), resulting in a decreased production of prostaglandins and thromboxanes; 2) inhibition of the lipoxygenase pathway, resulting in decreased production of leukotrienes, particularly the pro-inflammatory LKB4; and 3) inhibition of arachidonic acid release and stimulation of its reuptake, resulting in a reduction of arachidonic acid availability.Фармакокине?тика
Diclofenac is rapidly and completely (~100%) absorbed on oral administration, with peak plasma levels being reached within 1.5 to 2.5 hours. The free acid (pKa = 4.0) is highly bound to serum proteins (99.5%), primarily albumin. Only 50 to 60% of an oral dose is bioavailable because of extensive hepatic metabolism.Клиническое использование
Diclofenac is synthesized from N-phenyl-2,6-dichloroaniline. It is available in 120 different countries and, perhaps, is the most widely used NSAID in the world. It was introduced in the United States in 1989 but was first marketed in Japan in 1974. It ranks among the top prescription drugs in the United States. Diclofenac possesses structural characteristics of both arylalkanoic acid and the anthranilic acid classes of anti-inflammatory drugs, and it displays anti-inflammatory, analgetic, and antipyretic properties.Метаболизм
Four major metabolites resulting from aromatic hydroxylation have been identified. The major metabolite via CYP3A4 is the 4′-hydroxy derivative and accounts for 20 to 30% of the dose excreted, whereas the 5-hydroxy, 3′-hydroxy, and 4′,5-dihydroxy metabolites via CYP2C9 account for 10 to 20% of the excreted dose. The remaining drug is excreted in the form of sulfate conjugates. Although the major metabolite is much less active than the parent compound, it may exhibit significant biological activity, because it accounts for 30 to 40% of all of the metabolic products.1-(2,6-Dichlorophenyl)-2-indolinone запасные части и сырье
1-(2,6-Dichlorophenyl)-2-indolinone поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8617756083858 | China | 973 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +8615373025980 | China | 895 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8615531157085 | China | 8804 | 58 |