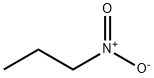
1-Нитропропан
- английское имя1-Nitropropane
- CAS №108-03-2
- CBNumberCB4852731
- ФормулаC3H7NO2
- мольный вес89.09
- EINECS203-544-9
- номер MDLMFCD00007407
- файл Mol108-03-2.mol
| Температура плавления | -108 °C |
| Температура кипения | 132 °C |
| плотность | 0.998 g/mL at 25 °C(lit.) |
| плотность пара | 3.1 (vs air) |
| давление пара | 7.5 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 93 °F |
| температура хранения | Store below +30°C. |
| растворимость | 14g/l |
| пка | pK1:8.98 (25°C) |
| форма | Liquid |
| цвет | Clear |
| РН | 6.0 (0.9g/l, H2O, 20℃) |
| Пределы взрываемости | 2.2-11.0%(V) |
| Растворимость в воде | 1.40 g/100 mL |
| Мерк | 14,6626 |
| БРН | 506236 |
| констант закона Генри | 6.11 at 25 °C (static headspace-GC, Welke et al., 1998) |
| Пределы воздействия | NIOSH REL: TWA 25 ppm (90 mg/m3), IDLH 1,000 ppm; OSHA PEL: TWA 25 ppm; ACGIH TLV: TWA 25 ppm (adopted). |
| Диэлектрическая постоянная | 23.239999999999998 |
| Стабильность | Stable. Flammable. Incompatible with strong bases, strong oxidizing agents. |
| ИнЧИКей | JSZOAYXJRCEYSX-UHFFFAOYSA-N |
| LogP | 0.79 at 22℃ |
| Справочник по базе данных CAS | 108-03-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | 39W305OW99 |
| Справочник по химии NIST | Propane, 1-nitro-(108-03-2) |
| Система регистрации веществ EPA | 1-Nitropropane (108-03-2) |
| UNSPSC Code | 12352102 |
| NACRES | NA.22 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 10-20/21/22 | |||||||||
| Заявления о безопасности | 9-24/25 | |||||||||
| РИДАДР | UN 2608 3/PG 3 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 25 ppm (90 mg/m3) | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TZ5075000 | |||||||||
| Температура самовоспламенения | 788 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29042000 | |||||||||
| Банк данных об опасных веществах | 108-03-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 455 mg/kg LD50 dermal Rabbit > 2000 mg/kg | |||||||||
| ИДЛА | 1,000 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H302+H312:Вредно при проглатывании или при попадании на кожу.
H331:Токсично при вдыхании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
1-Нитропропан химические свойства, назначение, производство
Описание
1-Nitropropane (1-NP), a representative small nitro-alkane, is widely used as an industrial solvent and a fuel additive in automobiles and aircraft engines. The improvement effects of 1-NP on fuel combustion demonstrate that 1-NP served as an initiator can rapidly produce a large number of reactive radicals, which significantly increases the fuel conversion rate. In addition, among the small nitro-alkanes, the molecular structure of 1-NP endows it with a higher specific impulse. It provides the possibilities for its application as propellant in long-range rocket systems[1].Химические свойства
Colorless liquid.Soluble in water 1.4 mL/100 mL (20C); solubility of water in 1-nitropropane 0.5 cc/100 cc (20C).Физические свойства
Colorless, oily liquid with a mild, fruity odor. A detection odor threshold concentration of 510 mg/m3 (140 ppmv) was experimentally determined by Dravnieks (1974).Использование
The solvent of 1-Nitropropane is mainly used coatings, inks and separation processes.Методы производства
1-Nitropropane is produced by vapor-phase nitration of propane with nitric acid (Baker and Bollmeier 1978) or by the vapor-phase nitration of butanol (HSDB 1988).Опасность
Flammable, moderate fire risk, moderate explosion hazard when shocked or heated. Liver damage, eye and upper respiratory tract irritant. Questionable carcinogen.Угроза здоровью
Humans exposed briefly to vapors of 1-nitropropane were found to have concentrations exceeding 100 p.p.m. irritating to the eyes (HSDB 1988). The chemical may produce anorexia, nausea, vomiting, diarrhea as well as injury to the liver and kidneys in exposed humans. High concentrations of the chemical also may produce methemoglobinemia with cyanosis. 1-Nitropropane vapors also are damaging to the lungs. 1-Nitropropane is, however, more acutely irritating to mucus membranes than 2-nitropropane. Despite this, the TLV for human exposure to 2-nitropropane is less than that for 1-nitropropane, based on the carcinogenic potential of 2-nitropropane in rats.Промышленное использование
1-Nitropropane is used as a solvent for cellulose acetate, vinyl resins and lacquers. It is also used as a gasoline additive (HSDB 1988).Профиль безопасности
Poison by ingestion and intraperitoneal routes. Mildly toxic by inhalation. A human eye irritant. Human systemic effects by inhalation: conjunctiva irritation. Mutation data reported. Very dangerous fire hazard when exposed to heat, open flame, or oxidizers. Reacts violently with Ca(OH)2, hydrocarbons, hydroxides, inorganic bases. May explode on heating. Metal oxides increase its sensitivity to thermal ignition. To fight fire, use alcohol foam, CO2, dry chemical, water spray. When heated to decomposition it emits toxic fumes of NOx. See also 2- NITROPROPANE, NITROALKANES, and NITRO COMPOUNDS.Возможный контакт
1-Nitropropane is used as a solvent for polymers, as a stabilizer; and in organic synthesis. Note: Technical products measurably contaminated with 2-Nitropropane, see also 2-Nitropropane (N: 0550)Метаболизм
1-Nitropropane is a substrate for the liver microsomal cytochrome P-450-dependent mixed-function oxidase system. Oxidative denitrification of 1-nitropropane by phenobarbital-induced rat microsomes occurred at a rate of 0.6 nmole/min/mg protein. This rate was much slower than for 2-nitropropane which was metabolized at 2.4 nmole/min/mg microsomal protein (Ullrich et al 1978).The role of the cytochrome P-450 system in vivo in 1-nitropropane metabolism is unknown. Bray and James (1958) isolated a small amount of a mercapturic acid metabolite from the urine of rabbits dosed with 1-nitropropane.
Перевозки
UN2608 Nitropropanes, Hazard Class: 3; Labels: 3-Flammable liquid.Методы очистки
Purify it as for nitromethane. [Beilstein 1 IV 229.]Несовместимости
1-Nitropropane, a nitroparaffinin, forms exposive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, reducing agents; nitrates, amines, hydrocarbons, and other combustible materials; metal oxides. May explode on heating.1-Нитропропан запасные части и сырье
1-Нитропропан поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +8618957127338 | China | 2136 | 58 | |
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| 18017061086 | CHINA | 5600 | 58 | |
| 18503026267 | CHINA | 9636 | 58 |
1-Нитропропан Обзор)
1of4