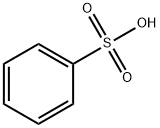
Бензолсульфокислота
- английское имяBenzenesulfonic acid
- CAS №98-11-3
- CBNumberCB4668758
- ФормулаC6H6O3S
- мольный вес158.18
- EINECS202-638-7
- номер MDLMFCD00011689
- файл Mol98-11-3.mol
| Температура плавления | 30-60 °C |
| Температура кипения | 137℃ |
| плотность | 1.32 |
| давление пара | 69.8Pa at 20℃ |
| показатель преломления | 1.5151 (estimate) |
| Fp | >230 °F |
| температура хранения | Store below +30°C. |
| растворимость | H2O: soluble0.1g/10 mL, clear, colorless |
| форма | Damp Crystalline Solid or Fused Mass |
| пка | 0.7(at 25℃) |
| цвет | Yellow to light brown |
| РН | 2 (H2O, 20℃)(saturated solution) |
| Вязкость | 166.2mm2/s |
| Биологические источники | synthetic |
| Растворимость в воде | soluble |
| Чувствительный | Hygroscopic |
| Мерк | 14,1070 |
| БРН | 742513 |
| Стабильность | Stable. Incompatible with strong oxidizing agents, bases, many organic compounds. |
| ИнЧИКей | SRSXLGNVWSONIS-UHFFFAOYSA-N |
| LogP | 0.41 at 25℃ |
| Справочник по базе данных CAS | 98-11-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 685928Z18A |
| Справочник по химии NIST | Benzenesulfonic acid(98-11-3) |
| Система регистрации веществ EPA | Benzenesulfonic acid (98-11-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.22 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 22-34 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-36 | |||||||||
| РИДАДР | UN 2583 8/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | DB4200000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29041000 | |||||||||
| Банк данных об опасных веществах | 98-11-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 1175 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Бензолсульфокислота химические свойства, назначение, производство
Описание
Benzene sulfonic acid is an organo sulfur compound with the formula C6H5SO3H. It is the simplest aromatic sulfonic acid. It forms colorless deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in carbon disulfide and diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic.Химические свойства
green solidИстория
Benzenesulfonic acid was first obtained, together with diphenyl sulfone, by E. M ITSCHERLICH in 1834 by heating benzene with fuming sulfuric acid. The industrially important reaction of benzenesulfonic acid with alkali hydroxide to form phenol (alkali fusion) was developed by A. WURTZ and A. KEKULé in 1867 and by P. O. D EGENER in 1878. Until the early 1960s benzenesulfonic acid was used chiefly in the manufacture of phenol. Other phenol syntheses are preferred now.Использование
Benzenesulfonic acid is mainly consumed by conversion to other specialty chemicals. A variety of pharmaceutical drugs are prepared as salts of benzenesulfonic acid and are known as besylates or besilates. The alkali metal salt of benzenesulfonic acid was once widely used in the production of phenol. It acts as an acid catalyst for direct esterification of amino acids and peptides.прикладной
The alkali metal salt of benzene sulfonic acid was once widely used in the production of phenol :C6H5SO3Na + 2 NaOH → C6H5ONa + Na2SO3
C6H5ONa + HCl → C6H5OH + NaCl
The process has been largely displaced by the Hock process, which generates less waste. Benzene sulfonic acid is mainly consumed by conversion to other specialty chemicals. A variety of pharmaceutical drugs are prepared as salts of benzene sulfonic acid and are known as besylates or besilates.
Подготовка
Benzene sulfonic acid is prepared from the sulfonation of benzene using concentrated sulfuric acid :This conversion illustrates aromatic sulfonation, which has been called "one of the most important reactions in industrial organic chemistry.".
прикладной
Benzenesulfonic acids are used chiefly as intermediates. They are employed in the manufacture of sulfonic acid amides, hydrazides, and esters; of sulfinic acids, sulfones, phenols, and thiophenols; and of other compounds. Sulfonic acids that are substituted with OH and/or NH 2 groups serve as intermediates in the manufacture of finishing agents, optical brighteners, pickling agents, dyes, tanning agents, water-soluble resins, insecticides, ion-exchange resins, wetting agents, pharmaceuticals, polymeric thickeners, plasticizers, etc. Benzenesulfonic acids are also used as such as acidic catalysts and standardizing agents in dye manufacture.Реакции
Benzene sulfonic acid exhibits the reactions typical of other aromatic sulfonic acids, forming sulfonamides , sulfonyl chloride, and esters. The sulfonation is reversed above 220 °C. Dehydration with phosphorus pentoxide gives benzene sulfonic acid anhydride ((C6H5SO2)2O). Conversion to the corresponding benzene sulfonyl chloride (C6H5SO2Cl) is effected with phosphorus penta chloride. It is a strong acid, being dissociated in water.Общее описание
Deliquescent needles or large plates.Реакции воздуха и воды
Slightly soluble in water.Профиль реактивности
Benzenesulfonic acid reacts with bases and many organic compounds. Benzenesulfonic acid has the characteristic reactions of a strong aromatic sulfonic acid. Acid hydrolysis at 175 ℃ splits it into benzene and sulfuricacid. Additional sulfonation with fuming sulfuric acid gives 1,3-benzenedisulfonic acid, which reacts further to 1,3,5-benzenetrisulfonic acid, and also diphenyl sulfone disulfonic acid.Benzenesulfonic acid reacts with benzene to form diphenyl sulfone according to a Friedel – Crafts-type reaction.
Benzenesulfonic acid reacts with alkali metal hydroxide at 320 – 350 ℃ to form sodium phe- nolate. This reaction was used in the first industrial synthesis of phenol.
Пожароопасность
Flash point data for Benzenesulfonic acid are not available, however Benzenesulfonic acid is probably combustible.Профиль безопасности
Poison by ingestion, sbn contact, and probably inhalation. A severe skin and eye irritant. See also SULFATES and SULFONATES.Методы очистки
Purify benzenesulfonic acid by dissolving it in a small volume of distilled H2O and stirring with slightly less than the theoretical amount of BaCO3. When effervescence is complete and the solution is still acidic, filter off the insoluble barium benzenesulfonate. The salt is collected and dried to constant weight in vacuo, then suspended in H2O and stirred with a little less than the equivalent (half mol.) of sulfuric acid. The insoluble BaSO4 (containing a little barium benzenesulfonate) is filtered off and the filtrate containing the free acid is evaporated in a high vacuum. The oily residue will eventually crystallise when completely anhydrous. A 32% commercial acid is allowed to fractionally crystallise at room temperature over P2O5 in a vacuum desiccator giving finally colourless deliquescent plates m 52.5o. The anhydrous crystalline acid is deliquescent and should be stored over anhydrous Na2SO4 in the dark and should be used in subdued sunlight as it darkens under sunlight. The main impurity is Fe which readily separates as the Fe salt in the early fractions [Taylor & Vincent J Chem Soc 3218 1952]. The S-benzylisothiuronium salt has m 148o (from EtOH/H2O). It is an IRRITANT to the skin and eyes. [See Adams & Marvel Org Synth Coll Vol I 84 1941, Michael & Adair Chem Ber 10 585 1877, Beilstein 11 IV 27.]Бензолсульфокислота запасные части и сырье
сырьё
запасной предмет
1of4
Бензолсульфокислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | |
| +86-371-66670886 | China | 19902 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 |