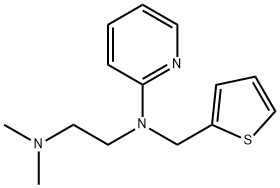
Метапирилен
- английское имяMETHAPYRILENE
- CAS №91-80-5
- CBNumberCB4500689
- ФормулаC14H19N3S
- мольный вес261.38576
- EINECS202-099-8
- номер MDLMFCD00242573
- файл Mol91-80-5.mol
химическое свойство
| Температура плавления | 25°C |
| Температура кипения | bp0.45 125-135°; bp3 173-175° |
| плотность | 1.1388 (rough estimate) |
| показатель преломления | nD25 1.5842 (also reported as 1.5835) |
| температура хранения | Refrigerator, under inert atmosphere |
| растворимость | DMSO (Sparingly), Methanol (Slightly) |
| форма | Oil |
| пка | pKa 3.02 ± 0.05;8.24± 0.11(H2O,t undefined,I=0.30(NaCl)) (Uncertain) |
| цвет | Colourless to Light Yellow |
| Растворимость в воде | 601.2mg/L(30 ºC) |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| FDA UNII | A01LX40298 |
| Код УВД | R06AC05 |
| Система регистрации веществ EPA | Methapyrilene (91-80-5) |
| РИДАДР | 1851 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Банк данных об опасных веществах | 91-80-5(Hazardous Substances Data) |
| Токсичность | LD50 in mice, guinea pigs (mg/kg): 182.2 ±12.8, 374.9 ±34.5 orally; in mice (mg/kg): 19.85 ±0.69 i.v. (Lee) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Метапирилен химические свойства, назначение, производство
Химические свойства
colourless liquidИспользование
Methapyrilene is an intermediate in the synthesis of Methapyrilene Hydrochloride (M259970). Methapyrilene is used as antihistaminic agent.Определение
ChEBI: A member of the class of ethylenediamine derivatives that is ethylenediamine in which one of the nitrogens is substituted by two methyl groups, and the other nitrogen is substituted by a 2-pyridyl group and a (2-thienyl)methyl group.Всемирная организация здравоохранения(ВОЗ)
Methapyrilene, an antihistamine with moderate sedative activity, was introduced in 1947 for the treatment of various allergic conditions and was subsequently incorporated in many over-the-counter sleeping aids. In the early 1970s it was identified as a carcinogen in rats and, although there was no direct evidence that it constitutes a health hazard to man, it was withdrawn in many countries. (Reference: (WHODI) WHO Drug Information, 2, 4, 1979)Общее описание
Clear colorless liquid.Профиль реактивности
An amine, organosulfide. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.Угроза здоровью
ACUTE/CHRONIC HAZARDS: METHAPYRILENE is highly toxic by ingestion.Пожароопасность
Flash point data for METHAPYRILENE are not available, but METHAPYRILENE is probably combustible.Метаболический путь
When methaphenilene is incubated with rat liver microsomes, it is metabolized via N-oxide formation and N-dealkylation which includes removal of the dimethylamino moiety, the thiophenylmethyl moiety of methaphenilene, and the benzyl moiety of pyribenzamine.Метапирилен запасные части и сырье
Метапирилен поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +8613564774135 | United States | 19881 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| 010-82848833 400-666-7788 |
China | 96815 | 76 | |
| 021-33632979 | China | 8065 | 58 | |
| 0755-0755-66853366 13670046396 |
China | 18097 | 56 | |
| +86-400-002-6226 +86-13028896684; |
China | 57423 | 58 | |
| 020-82000279 18988968278 |
China | 4297 | 58 | |
| 18017382783 18017382783 |
China | 9611 | 58 |