Chemical Properties
colourless liquid
Uses
antihypertensive, AT1 angiotensin II antagonist
Definition
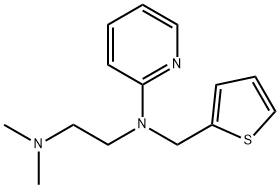
ChEBI: A member of the class of ethylenediamine derivatives that is ethylenediamine in which one of the nitrogens is substituted by two methyl groups, and the other nitrogen is substituted by a 2-pyridyl group and a (2-thienyl)methyl group.
General Description
Clear colorless liquid.
Reactivity Profile
An amine, organosulfide. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: METHAPYRILENE is highly toxic by ingestion.
Fire Hazard
Flash point data for METHAPYRILENE are not available, but METHAPYRILENE is probably combustible.
Originator
Thenylene ,Abbott,US,1947
Manufacturing Process
To a slurry of sodamide in 200 cc of toluene representing 6.7 g of sodium was added at 30° to 40°C, 32.3 g (0.31 mol) of 2-aminopyridine. The mixture was heated to reflux temperature and was refluxed for 1? hours. To the resulting mixture was added over a period of approximately one hour a solution of 32 g of freshly distilled N,N-dimethyl-β-chloroethylamine in 40 to 50 cc of dry toluene, The reaction mixture was then heated for 2 hours at reflux temperature. Thereafter, 200 cc of water was added and the toluene layer was separated and washed with water. The toluene was stripped from the mixture by distillation and the residue was distilled under reduced pressure. The distillate was refractionated and the portion distilled at 93° to 103°C/1 mm was recovered. Yield of N-(2-pyridyl)-N',N'-dimethyl-ethylenediamine, 60%.
A solution of 20 g (0.121 mol) of N-(2-pyridyl)-N',N'-dimethylethylenediamine in 25 cc of toluene was added to a slurry of sodamide in 100 cc of toluene representing 2.8 g of sodium. The mixture was refluxed for one hour. To this mixture was added over a period of ? hour a solution of 16 g (0.121 mol) of 2-thenyl chloride in 25 cc of toluene. The resulting reaction mixture was refluxed for 3 hours. Thereafter, water was added and the toluene layer was separated and washed with water.
The toluene was then stripped off by distillation and the residue was distilled under reduced pressure. The main fraction was redistilled. Yield of N-(2- pyridyl)-N-(2-thenyl)-N',N'-dimethyl-ethylenediamine was 69%; BP 130° to 140°C/0.4 mm. A portion of the product was dissolved in ether and an ether solution of hydrogen chloride was added. The monohydrochloride of N-(2- pyridyl)-N-(2-thenyl)-N',N'-dimethyl-ethylenediamine which separated was washed with ether and dried.
Brand name
3p pane;Brexin;Conac;Dexapirilene;Dormin;Duohist;Duo-tussin;Dylhista;Histadyl ec;Hitalones;Isopap;Lallamin;Lullamin;M.p.;Methistaline;Methril spansul;M-p;Myci-spray;Norane;Paradormalene;Peral;Placitabs;Pyrathyn;Pyrinistab;Pyrinistol;Rejam;Thenylene;Thionylan;W83.
Therapeutic Function
Antihistaminic
World Health Organization (WHO)
Methapyrilene, an antihistamine with moderate sedative activity,
was introduced in 1947 for the treatment of various allergic conditions and was
subsequently incorporated in many over-the-counter sleeping aids. In the early
1970s it was identified as a carcinogen in rats and, although there was no direct
evidence that it constitutes a health hazard to man, it was withdrawn in many
countries.
(Reference: (WHODI) WHO Drug Information, 2, 4, 1979)
Synthesis
Methapyrilene is synthesized by heating a
2-thienyl halide with an alkali metal salt
of N,N-dimethyl-N_x0002_-(2-pyridinyl)-1,2-ethanediamine
.
Metabolic pathway
When methaphenilene is
incubated with rat liver microsomes, it is metabolized via N-oxide formation and
N-dealkylation which includes removal of the
dimethylamino moiety, the thiophenylmethyl moiety of
methaphenilene, and the benzyl moiety of
pyribenzamine.