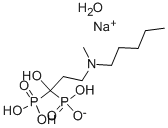
Ibandronate sodium monohydrate
- русский язык имя
- английское имяIbandronate sodium monohydrate
- CAS №138926-19-9
- CBNumberCB4286017
- ФормулаC9H24NNaO8P2
- мольный вес359.23
- EINECS639-757-2
- номер MDLMFCD00912167
- файл Mol138926-19-9.mol
химическое свойство
| Температура плавления | 840C (dec) |
| температура хранения | Keep in dark place,Inert atmosphere,2-8°C |
| растворимость | Soluble in DMSO (up to at least 25 mg/ml) |
| форма | solid |
| цвет | White |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| Справочник по базе данных CAS | 138926-19-9(CAS DataBase Reference) |
| FDA UNII | J12U072QL0 |
| Словарь наркотиков NCI | Boniva |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 40 | |||||||||
| Заявления о безопасности | 22-36-24/25 | |||||||||
| кода HS | 29319090 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Ibandronate sodium monohydrate химические свойства, назначение, производство
Описание
Ibandronate Sodium Monohydrate is a highly potent nitrogen-containing bisphosphonate used for the treatment of osteoporosis.Ibandronate Sodium Monohydrate inhibits bone resorption as a sodium salt and complexed with technetium Tc 99m for bone imaging. The monophosphonates are not active and the biphosphonates are used in disorders affecting the skeleton such as metastatic disease, asteoporosis and Paget's. Ibandronate Sodium Monohydrate is an inhibitor of FDPS.
Химические свойства
[1-Hydroxy-3-(methylpentylamino)-propylidene]bisphosphonic acid sodium salt is White Crystalline PowderИспользование
Inhibits bone resorption as a sodium salt and complexed with technetium Tc 99m for bone imaging. The monophosphonates are not active. Biphosphonates are used in disorders affecting the skeleton such as asteoporosis, metastatic disease, and Paget dМеханизм действия
The action of ibandronate on bone tissue is based on its affinity for hydroxyapatite, which is part of the mineral matrix of bone. Ibandronate inhibits osteoclast activity and reduces bone resorption and turnover. In postmenopausal women, it reduces the elevated rate of bone turnover, leading to, on average, a net gain in bone mass.Фармакокине?тика
AbsorptionThe absorption of oral ibandronate occurs in the upper gastrointestinal tract. Plasma concentrations increase in a dose-linear manner up to 50 mg oral intake and increases nonlinearly above this dose.
Following oral dosing, the time to maximum observed plasma ibandronate concentrations ranged from 0.5 to 2 hours (median 1 hour) in fasted healthy postmenopausal women. The mean oral bioavailability of 2.5 mg ibandronate was about 0.6% compared to intravenous dosing. The extent of absorption is impaired by food or beverages (other than plain water). The oral bioavailability of ibandronate is reduced by about 90% when BONIVA is administered concomitantly with a standard breakfast in comparison with bioavailability observed in fasted subjects. There is no meaningful reduction in bioavailability when ibandronate is taken at least 60 minutes before a meal. However, both bioavailability and the effect on bone mineral density (BMD) are reduced when food or beverages are taken less than 60 minutes following an ibandronate dose.
Distribution
After absorption, ibandronate either rapidly binds to bone or is excreted into urine. In humans, the apparent terminal volume of distribution is at least 90 L, and the amount of dose removed from the circulation via the bone is estimated to be 40% to 50% of the circulating dose. In vitro protein binding in human serum was 99.5% to 90.9% over an ibandronate concentration range of 2 to 10 ng/mL in one study and approximately 85.7% over a concentration range of 0.5 to 10 ng/mL in another study.
Metabolism
There is no evidence that ibandronate is metabolized in humans.
https://www.accessdata.fda.gov
Ibandronate sodium monohydrate запасные части и сырье
сырьё
Ibandronate sodium monohydrate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 28-85305008 | CHINA | 964 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-371-66670886 | China | 19902 | 58 | ||
| 010-60279497 | CHINA | 1803 | 55 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | ||
| 86-13657291602 | CHINA | 22963 | 58 | ||
| 86-714-3992388 | United States | 14332 | 58 |