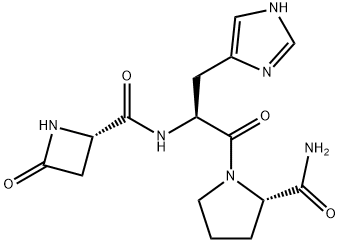
Азетирелин
- английское имяAzetirelin
- CAS №95729-65-0
- CBNumberCB41178074
- ФормулаC15H20N6O4
- мольный вес348.36
- номер MDLMFCD00867196
- файл Mol95729-65-0.mol
химическое свойство
| Температура плавления | >125°C (dec.) |
| Температура кипения | 997.1±65.0 °C(Predicted) |
| плотность | 1.469±0.06 g/cm3(Predicted) |
| температура хранения | -20°C Freezer, Under inert atmosphere |
| растворимость | DMSO (Slightly), Methanol (Slightly), Water (Very Slightly, Heated) |
| форма | Solid |
| пка | 13.98±0.40(Predicted) |
| цвет | Off-White |
| FDA UNII | 70J5AWG54Q |
Азетирелин химические свойства, назначение, производство
Создатель
Azetirelin,ZYF Pharm ChemicalПроизводственный процесс
In 13 ml of DMF was dissolved 826.0 mg of L-histidyl-L-prolinamide 2- hydrobromide and then 2 ml of a DMF solution of 405.0 mg of triethylamine was added to the solution under ice-cooling. After maintaining 30 min under ice-cooling, the precipitates thus formed were filtered off to provide L-histidyl- L-prolinamide.In 10 ml of DMF was dissolved 230.0 mg of (S)-2-azetidinone-4-carboxylic acid and then 351.0 mg of N-hydroxy-1,2,3-benzotriazole and 453.0 mg of dicyclohexylcarbodiimide were added to the solution under ice-cooling. Then, after stirring the mixture for 15 min, the reaction was maintained for 15 min at room temperature. The reaction mixture was ice-cooled again and 15 ml of a DMF solution of foregoing L-histidyl-L-prolinamide was added to the reaction mixture followed by reaction overnight at 0°C. The precipitates thus formed were filtered off, the filtrate was concentrated to dryness, the residue was dissolved in 10 ml of chloroform-methanol (4:1) and subjected to silica gel column chromatography. The eluates by chloroform-methanol (7:3) were collected and concentrated to dryness to provide 509.0 mg of crude product. When the product was subjected to silica gel column chromatography again and eluted by a mixture of chloroform, methanol, and aqueous ammonia (40:10:1) to provide 394.0 mg of pure Nepsilon-[(S)-2-azetidinone-4-carbonyl]- L-histidyl-L-prolinamide, melting point 183°-185°C (crystallization from a small amount of methanol).
Терапевтическая функция
TRH analogueАзетирелин запасные части и сырье
Азетирелин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| United States | 38631 | 58 | ||
| +8613564774135 | United States | 19881 | 58 | |
| 025-57672321 13739186949 |
China | 298 | 58 | |
| 15002134094 | China | 19699 | 58 |