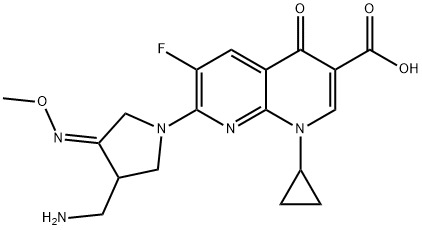
Гемифлоксацин
- английское имяGemifioxacin
- CAS №175463-14-6
- CBNumberCB3855593
- ФормулаC18H20FN5O4
- мольный вес389.38
- номер MDLMFCD07779399
- файл Mol175463-14-6.mol
химическое свойство
| Температура плавления | 235-237° |
| Температура кипения | 638.9±65.0 °C(Predicted) |
| плотность | 1?+-.0.1 g/cm3(Predicted) |
| пка | 6.02±0.70(Predicted) |
| Справочник по базе данных CAS | 175463-14-6 |
| FDA UNII | OKR68Y0E4T |
| Код УВД | J01MA15 |
| Банк данных об опасных веществах | 175463-14-6(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Гемифлоксацин химические свойства, назначение, производство
Описание
LG Life Sciences (formerly LG Chemical) has developed gemifloxacin (SB-265805, LB-20304a), a fluoronaphthyridone active against both Gram-positive and Gram-negative bacteria, including methicillin-resistant staphylococci, as a treatment for bacterial infection. By December 2002, the drug had been approved in Korea.Определение
ChEBI: A 1,4-dihydro-1,8-naphthyridine with a carboxy group at the 3-position, an oxo sustituent at the 4-position, a fluoro substituent at the 5-position and a substituted pyrrolin-1-yl group at the 7-position.Антимикробная активность
The broad antibacterial spectrum embraces most Gram-positive cocci (including high potency against Str. pneumoniae) and Gram-negative bacilli. It possesses a high affinity for pneumococcal topoisomerase IV. Activity against Gram-negative respiratory tract pathogens such as H. influenzae, Mor. catarrhalis, Ch. pneumoniae, L. pneumophila and Mycoplasma pneumonia is good. It is relatively inactive against Ps. aeruginosa and Enterococcus spp. Activity against Enterobacteriaceae is similar to that of moxifloxacin but it is less potent against anaerobes. Gemifloxacin is inactive against M. tuberculosis. Activity against Nocardia asteroides (MIC 0.5–1 mg/L) is better than that of other quinolones other than the investigational compound nemonoxacin .Multistep resistance studies suggest that it is less likely than other quinolones to select for quinolone-resistant Str. pneumoniae strains. Because it inhibits both DNA gyrase and DNA topoisomerase IV enzyme systems at therapeutically relevant drug levels in Str. pneumoniae, single mutations in parC or gyrA result in only a small increase in the MIC. In Str. pneumoniae gyrA mutations arise at a lower rate (1.6 × 10?11) than mutations in parC. It seems to be unaffected clinically by quinolone efflux mechanisms in Str. pneumoniae. Low rates of resistance selection have also been reported in H. influenzae.
Фармацевтические приложения
A fluoronaphthyridone derivative with a dual substituted pyrrolidine moiety at the C-7 position. It is formulated as the mesylate.Фармакокине?тика
absorption and distributionIn oral escalating dose studies (single doses of 20–800 mg), Cmax ranged from 0.12 to 4.33 mg/L after an average of 1 h. Antacids significantly reduce the systemic availability and protein binding is relatively high. Excellent concentrations are achieved in serum as well as various tissues such as bronchial mucosa, epithelial lining fluid and alveolar macrophages. Absolute bioavailability of the 320 mg oral tablet is around 71%. Pharmacokinetics are not significantly altered when administered with a high fat meal.
Metabolism and excretion
The apparent elimination half-life ranges from 6 to 9 h, and 26–40% of administered doses are eliminated in urine. It is metabolized to a limited extent in the liver. Cytochrome P450 enzymes do not play an important role in metabolism, and the metabolic activity of these enzymes is unaffected. Around 65% of the parent compound and its metabolites are eliminated in the feces and the remainder in the urine. The mean renal clearance after repeated doses of 320 mg is about 11.6 L/h, indicating active renal secretion. The mean apparent elimination half-life at steady state following administration of 320 mg to healthy subjects was approximately 7 h. No dosage adjustment is recommended in patients with mild, moderate or severe hepatic impairment. Clearance is reduced and plasma elimination is prolonged in patients with renal insufficiency, leading to an average increase in AUC values of c. 70%. Hemodialysis removes approximately 20–30% of an oral dose from plasma.
Клиническое использование
Community-acquired pneumonia in adultsAcute exacerbations of chronic bronchitis in adults
Побочные эффекты
The most commonly reported side effects are diarrhea (3.6%), rash (2.8%) and nausea (2.7%). No evidence has emerged of a clinically significant prolongation in QTc interval. The phototoxicity potential is low and similar to that seen with ciprofloxacin. The overall incidence of drugrelated rash is 2.8%. The rash is most commonly mild, macropapular (occasionally urticarial), predominantly selflimiting, and mainly occurs in women under 40 years and in postmenopausal women on hormone replacement therapy after ≥10 days.Гемифлоксацин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +8618058761490 | China | 49983 | 58 | |
| +8618950047208 | China | 43416 | 58 |