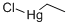
ETHYLMERCURIC CHLORIDE
- русский язык имя
- английское имяETHYLMERCURIC CHLORIDE
- CAS №107-27-7
- CBNumberCB3417751
- ФормулаC2H5ClHg
- мольный вес265.1
- EINECS203-478-0
- номер MDLMFCD00013593
- файл Mol107-27-7.mol
химическое свойство
| Температура плавления | 195°C |
| плотность | 3.482 |
| растворимость | Chloroform (Slightly) |
| форма | solid |
| Растворимость в воде | Insoluble in water. Slightly soluble in ether. Soluble in hot alcohol. Sublimes readilyInsoluble in water. Slightly soluble in ether. Soluble in hot alcohol. |
| Мерк | 14,3826 |
| Пределы воздействия | NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. May be light sensitive. |
| Справочник по базе данных CAS | 107-27-7(CAS DataBase Reference) |
| FDA UNII | M04218TP6P |
| Система регистрации веществ EPA | Mercury, chloroethyl- (107-27-7) |
| Коды опасности | T+,N | |||||||||
| Заявления о рисках | 26/27/28-33-50/53 | |||||||||
| Заявления о безопасности | 13-28-36-45-61-60 | |||||||||
| РИДАДР | 2025 | |||||||||
| RTECS | OV9800000 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28521000 | |||||||||
| Токсичность | mammal (species unspecified),LD50,unreported,18mg/kg (18mg/kg),"Chemistry of Pesticides," Melnikov, N.N., New York, Springer-Verlag New York, Inc., 1971Vol. -, Pg. 287, 1971. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
вредная бумага
H400:Чрезвычайно токсично для водных организмов.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H330:Смертельно при вдыхании.
H310:Смертельно при попадании на кожу.
H300:Смертельно при проглатывании.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P405:Хранить в недоступном для посторонних месте.
ETHYLMERCURIC CHLORIDE химические свойства, назначение, производство
Химические свойства
silver crystals or white solidИспользование
Applied at 2% strength (soln or mixed with solids) as a fungicide for treating seeds.Общее описание
Silver iridescent crystals or white fluffy solid . Sublimes easily. Sensitive to light. Highly toxic. Causes skin burns and is absorbed through the skin.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
ETHYLMERCURIC CHLORIDE is sensitive to prolonged exposure to light. Incompatible with strong oxidizers such as chlorine .Опасность
Strong irritant.Пожароопасность
Flash point data for ETHYLMERCURIC CHLORIDE are not available; however, ETHYLMERCURIC CHLORIDE is probably combustible.Профиль безопасности
Poison by ingestion, inhalation, skin contact, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. See also MERCURY COMPOUNDS, ORGANIC. When heated to decomposition it emits very toxic fumes of Cland Hg.Возможный контакт
This chemical is used in manufacture of coatings, resins, and lacquers. Widely known as “Plexiglass” (in the polymer form), ethyl methacrylate is used to make polymers, which in turn are used for building, automotive, aerospace, and furniture industries. It is also used by dentists as dental plates, artificial teeth, and orthopedic cement.Перевозки
UN2277 Ethyl methacrylate, stabilized, Hazard Class: 3; Labels: 3-Flammable liquidМетоды очистки
Mercuric chloride can be removed by suspending ethylmercuric chloride in hot distilled water, filtering with suction onto a sintered-glass crucible and drying it. Then crystallise it from ethanol and sublime it under reduced pressure. It can also be crystallised from water. [Marvel et al. J Am Chem Soc 47 3009 1925.]Несовместимости
May form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine,etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Corrodes some metals. Unless inhibited, violent polymerization can occur from heat, sunlight, and contact with strong oxidizersУтилизация отходов
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.