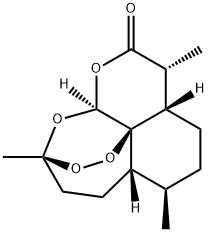
Артемизинин
- английское имяArtemisinin
- CAS №63968-64-9
- CBNumberCB3387975
- ФормулаC15H22O5
- мольный вес282.33
- EINECS1806241-263-5
- номер MDLMFCD00081057
- файл Mol63968-64-9.mol
| Температура плавления | 156-157 °C (lit.) |
| альфа | 76 º (c=0.5,MeOH) |
| Температура кипения | 344.94°C (rough estimate) |
| плотность | 1.0984 (rough estimate) |
| показатель преломления | 75 ° (C=0.5, MeOH) |
| температура хранения | -20°C |
| растворимость | Soluble to 100mM in DMSO and to 75mM in ethanol |
| форма | White to off-white crystalline solid. |
| цвет | Needles |
| оптическая активность | [α]20/D +76°, c = 0.5 in methanol |
| Мерк | 14,817 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides. May absorb, and react with, carbon dioxide from the air. |
| InChI | InChI=1S/C15H22O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8-11,13H,4-7H2,1-3H3/t8-,9-,10+,11+,13-,14-,15-/m1/s1 |
| ИнЧИКей | BLUAFEHZUWYNDE-NNWCWBAJSA-N |
| SMILES | O1[C@]23[C@@]4([H])O[C@@](C)(CC[C@@]2([H])[C@H](C)CC[C@@]3([H])[C@@H](C)C(=O)O4)O1 |
| LogP | 2.900 |
| FDA UNII | 9RMU91N5K2 |
| Код УВД | P01BE01 |
| UNSPSC Code | 41116107 |
| NACRES | NA.79 |
| Заявления о безопасности | 22-24/25 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | KD4170000 | |||||||||
| кода HS | 29322985 | |||||||||
| Токсичность | LD50 in mice (mg/kg): 5105 orally; 2800 i.m.; 1558 i.p. (Koch); LD50 in mice, rats (mg/kg): 4228, 5576 orally; 3840, 2571 i.m. (China Cooperative Research Group on Qinghaosu) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H400:Чрезвычайно токсично для водных организмов.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H242:При нагревании возможно возгорание.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P220:Не допускать соприкосновения с одеждой и другими горючими материалами.
P234:Хранить только в оригинальной упаковке.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P391:Ликвидировать просыпания/проливы/утечки.
P410:Беречь от солнечных лучей.
P420:Хранить отдельно.
Артемизинин химические свойства, назначение, производство
Описание
Artemisinin, a sesquiterpene isolated from a traditional Chinese remedy (quinghao), is useful in the treatment of Fafciparum malaria, including infections caused by chloroquine resistant strains. It is reported to clear parasitemia quicker than i.v. quinine, and is effective in cerebral malaria.Химические свойства
Crystalline SolidФизические свойства
Appearance: colorless needles or white crystalline powder. Solubility: practically insoluble in water, very soluble in dichloromethane, freely soluble in acetone and ethyl acetate, and soluble in glacial acetic acid, methanol, and ethanol. Melting point: 150–153?°C. Specific optical rotation: +75 to +78°.История
The discovery of artemisinin dramatically changes the landscape to combat malaria and leads to a paradigm shift in antimalarial drug development.However, the discovery of artemisinin is the first stage; the development of artemisinin derivatives and their compound preparations is another important stage. Based on artemisinin, scientists obtained artemisinin ether derivatives by semisynthetic method. After screening of antimalarial activity, artemether was found. To further improve the solubility of artemisinin derivatives, artesunate was also found. The discovery of artesunate makes artemisinin and its derivatives much easier to promote, and more convenient dosage forms to treat malaria enriched the clinic application of artemisinin and its derivatives .
Использование
Artemisinin inhibits angiogenesis by down-regulating HIF-1α and VEGF expression in mouse embryonic stem cells. Artemisinin crosses the blood-brain barrier and is an inhibitor of human NOS2 (iNOS).Показания
Clinically, artemisinin is mainly used to treat malaria symptoms, malignant cerebral malaria, uncomplicated malaria, and severe malaria. Combined with different antimalarial can delay and prevent resistance of malaria parasites. In additional, artemisinin can also be used for systemic lupus erythematosus or discoid lupus erythematosus. Currently, artemisinin derivatives and their compound preparations are widely used in clinic.Определение
ChEBI: A sesquiterpene lactone obtained from sweet wormwood, Artemisia annua, which is used as an antimalarial for the treatment of multi-drug resistant strains of falciparum malaria.Антимикробная активность
Artemisinins are active against the erythrocytic and gametocyte stages of chloroquine-sensitive and chloroquine-resistant strains of P. falciparum and other malaria parasites. Two anomers of artemether are produced on synthesis, α-artemether and β-artemether, of which the latter has higher antimalarial activity. Activity against the protozoa Tox. gondii and Leishmania major and the helminth Schistosoma mansoni has been demonstrated in experimental models.Приобретенная устойчивость
Resistance caused, for example, by changes in the plasmodial endoplasmic reticulum ATPase has been shown in experimental models. There have been clinical reports of reduced susceptibility to treatment with artesunate in Cambodia.Общее описание
The artemisinin series are the newest of the antimalarialdrugs and are structurally unique when comparedwith the compounds previously and currently used. Theparent compound, artemisinin, is a natural product extractedfrom the dry leaves of Artemisia Annua (sweetwormwood). The plant has to be grown each year fromseed because mature plants may lack the active drug. The growing conditions are critical to maximize artemisininyield. Thus far, the best yields have been obtained fromplants grown in North Vietnam, Chongqing province inChina, and Tanzania.Фармацевтические приложения
Artemisinin (qinghaosu), a compound derived from a plant used in traditional Chinese medicine, Artemisia annua, has been used extensively in East Asia and Africa for the treatment of malaria. This drug, and derivatives that have higher intrinsic antimalarial activity (artesunate, artemether and arteether), have replaced quinine as a treatment of falciparum malaria in many countries, normally in combination with other antimalarials. A semisynthetic derivative, artemisone, which has higher efficacy than artesunate and lower toxicity potential, is in development. Artemisinin and its derivatives also show broad antiprotozoal, anthelmintic and antiviral activities.The novel structure, containing an endoperoxide bridge, has stimulated the development of semisynthetic and synthetic dioxane, trioxane and tetroxane compounds with activity against Plasmodium spp. and Schistosoma spp. Some of these synthetic trioxalanes are now in clinical development with Medicines for Malaria Venture and other organizations.
Биологическая активность
Antimalarial agent; interacts with heme to produce carbon-centred free radicals, causes protein alkylation and damages parasite microorganelles and membranes. Also selectively inhibits the P-type ATPase (PfATP6) of Plasmodium falciparum (K i ~ 150 nM). Displays antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies.Фармакокине?тика
Oral absorption: IncompleteCmax 500 mg oral: 0.4 mg/L after 1.8 h
Plasma half-life (dihydroartemisinin): 40–60 min
Volume of distribution: c. 0.25 L/kg
Plasma protein binding (artemether): 77%
Artemisinins are concentrated by erythrocytes and are rapidly hydrolyzed to dihydroartemisinin. They are hydroxylated by cytochromes 2B6, 2C19 and 3A4; the derivatives induce this metabolism. After injection, peak plasma concentrations are reached within 1–3 h, when levels of dihydroartemisinin are included. The elimination half-life of intravenous artesunate is <30 min; artemether appears to have a much longer half-life (4–11 h).
Фармаколо?гия
The mechanism of artemisinins is not known, but the most widely accepted theory is that they are first activated through cleavage after reacting with haem and iron(II) oxide, which results in the generation of free radicals that in turn damage susceptible proteins, resulting in the death of the parasite .Artemisinin and its derivatives also show a good antitumor effect , which is mainly via (1) apoptosis, ferroptosis, or necrosis; (2) anti-angiogenesis; (3) oxidative stress; (4) tumor suppressor genes; and (5) protein targeting. In addition, artemisinin can exhibit antiarrhythmic, anti-fibrotic, and immunomodulating effects.
Клиническое использование
Malaria (including cerebral malaria), in combination with other antimalarials.Побочные эффекты
A few toxic effects in addition to drug-induced fever and a reversible decrease in reticulocyte counts have been reported. High-dose studies in animal models show neurotoxicity and reproducible dose-related neuropathic lesions; dihydroartemisinin is a toxic metabolite but the precise causes of neurotoxicity are not clear. Embryotoxicity of artemisinin and derivatives has been reported in rodent and primate models, probably due to depletion of erythroblasts.Профиль безопасности
Moderately toxic by ingestion,intramuscular, and intraperitoneal routes. When heated todecomposition it emits acrid smoke and fumes.Артемизинин запасные части и сырье
Артемизинин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined18602966907 | China | 997 | 58 | ||
| +8618007136271 | China | 5999 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-18565342920; +8618565342920 |
China | 290 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 |