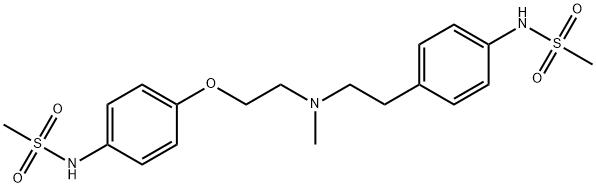
Дофетилид
- английское имяDofetilide
- CAS №115256-11-6
- CBNumberCB3381832
- ФормулаC19H27N3O5S2
- мольный вес441.56
- EINECS638-817-5
- номер MDLMFCD00869707
- файл Mol115256-11-6.mol
| Температура плавления | 147-1490C |
| Температура кипения | 614.1±65.0 °C(Predicted) |
| плотность | 1.344±0.06 g/cm3(Predicted) |
| температура хранения | 2-8°C |
| растворимость | DMSO: >20mg/mL |
| пка | 7.0, 9.0, 9.6(at 25℃) |
| форма | powder |
| цвет | white to off-white |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| ИнЧИКей | IXTMWRCNAAVVAI-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 115256-11-6(CAS DataBase Reference) |
| FDA UNII | R4Z9X1N2ND |
| Код УВД | C01BD04 |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | T,N | |||||||||
| Заявления о рисках | 61-48/22-51 | |||||||||
| Заявления о безопасности | 53-22-36-57 | |||||||||
| WGK Германия | nwg | |||||||||
| кода HS | 2935904000 | |||||||||
| Банк данных об опасных веществах | 115256-11-6(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H360:Может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Дофетилид химические свойства, назначение, производство
Описание
Dofetilide was launched in the US as a novel class III antiarrhythmic for treatment of cardiac patients with highly symptomatic atrial fibrillation This bisarylsulfonamide can be obtained by a three step synthesis starting from 4-nitro-N-methylphenethylamine and involving simultaneous nitro reduction and mesylation on both aromatic amine functions. In contrast to other class III antiarrhythmic agents such as amiodarone, dofetilide potently and selectively inhibits a single potassium channel, Ikr, the rapidly acting component of the delayed rectifier potassium current, Accordingly, by blocking the open state of Ikr, dofetilide is able to prolong the effective refractory period (ERP) in both atrial and ventricular myocardium and the monophasic action potential duration. Moreover, as it targets only one cardiac ion channel, it does not produce any effects on the sinus node, cardiac conduction system and other extracardiac organs, making it unique among established class III agents. Several pharmacological studies with models using different animal species indicated that dofetilide was a potent and highly selective class III antiarrhythmic agent devoid of cardiodepressive effects. During clinical trials in patients with paroxysmal atrial or supraventricular fibrillation, dofetilide was found to increase atrial and ventricular refractory periods without affecting conduction or sinus node function. If increases in the QT/QTc interval after oral or intravenous dofetilide are expected, as for other class III antiarrhythmic agents, other electrocardiographic intervals are unaffected.Химические свойства
White Crystalline SolidИспользование
Dofetilide (90%) , is a potassium channel blocker. Antidysrhythmic drug.Определение
ChEBI: A tertiary amino compound that is N-ethyl-N-methylethanamine substituted by a 4-[(methylsulfonyl)amino]phenoxy and a 4-[(methylsulfonyl)amino]phenyl group at the terminal carbon atoms respectively. It is used as an an i-arrhythmia drug.Общее описание
Dofetilide, N-[4-(3-{[2-(4-methanesulfonylaminophenyl)ethyl]methylamino}propoxy)phenyl]methane-sulfonamide (Tikosyn), acts by blocking thecardiac ion channel carrying the rapid component of thedelayed rectifier potassium currents (Ikr) and is used toterminate supraventricular arrhythmias, prevent the recurrenceof atrial fibrillation, and treat life-threatening ventriculararrhythmias. Unlike sotalol and ibutilide, whichare also methanesulfonanilides, it has no effect on adrenergicreceptors or sodium channels, respectively. Dofetilidehas high specificity for the delayed rectifier potassiumcurrents.Биологическая активность
Selective potassium channel blocker. Blocks hERG K + channels; inhibits the rapid delayed-rectifier K + current (I Kr ). Displays class III antiarrhythmic properties.Клиническое использование
Dofetilide (Tikosyn) is a “pure” class III drug. It prolongs the cardiac action potential and the refractory period by selectively inhibiting the rapid component of the delayed rectifier potassium current (IKr).Dofetilide is approved for the treatment of atrial fibrillation and atrial flutter. Because of the lack of significant hemodynamic effects, dofetilide may be useful in patients with CHF who are in need of therapy for supraventricular tachyarrhythmias. Dofetilide is not indicated for use in the setting of ventricular arrhythmias.
Побочные эффекты
The incidence of noncardiac adverse events is not different from that of placebo in controlled clinical trials. The principal cardiac adverse effect is the risk of torsades de pointes due to QT prolongation.The risk is approximately 3%, and most cases are observed in the first 3 days of therapy.As such, initiation of therapy should be performed with the patient in hospital.взаимодействия лекарств
Verapamil increases serum dofetilide levels, as do drugs that inhibit cationic renal secretion, such as ketoconazole and cimetidine, raise serum levels.Метаболизм
Dofetlide is used orally to suppress atrial fibrillation and flutter. It is more potent and selective than other Class III methanesulfonanilides, including sotalol. Dofetilide is well absorbed from the gastrointestinal tract, with a bioavailability of 96 to 100%. The bioavailability of oral dofetilide is not affected by food or antacids. Protein binding is 60 to 70%. Dofetilide is metabolized by the hepatic CYP3A4 enzyme system via N-dealkylation and N-oxidation to inactive or minimally active metabolites. Of the approximately 80% of a dose excreted in urine, approximately 80% is excreted unchanged, with the other 20% as metabolites.Меры предосторожности
Contraindications include baseline prolongation of the QT interval, use of other QT-prolonging drugs; history of torsades de pointes; a creatinine clearance of less than 20 mL/minute; simultaneous use of verapamil, cimetidine, or ketoconazole; uncorrected hypokalemia or hypomagnesemia; and pregnancy or breast-feeding.Дофетилид запасные части и сырье
сырьё
- арбонат калия
- 1- (2-хлорэтокси) -4-нитробензол
- BenzeneethanaMine, N-Methyl-4-nitro-
- Алюминия-никеля
- Dofetilide N`-Nitryl Impurity
1of4
Дофетилид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +undefined18602966907 | China | 997 | 58 | |
| +8619956560829 | China | 286 | 58 | |
| 010-60279497 | CHINA | 1803 | 55 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +8618957127338 | China | 2136 | 58 |