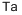
Тантал
- английское имяTantalum
- CAS №7440-25-7
- CBNumberCB3367656
- ФормулаTa
- мольный вес180.95
- EINECS231-135-5
- номер MDLMFCD00011252
- файл Mol7440-25-7.mol
| Температура плавления | 2996 °C (lit.) |
| Температура кипения | 5425 °C (lit.) |
| Плотность накопления | 500kg/m3 |
| плотность | 16.69 g/cm3 (lit.) |
| давление пара | <0.01 mm Hg ( 537.2 °C) |
| температура хранения | no restrictions. |
| растворимость | reacts with HF |
| форма | wire |
| цвет | Gray to silver |
| Удельный вес | 16.6 |
| РН | 7 (20°C in H2O, aqueous suspension) |
| Flame Color | Blue |
| удельное сопротивление | 13.5 μΩ-cm, 20°C |
| Растворимость в воде | very resistant to attack by acids except HF, resistant to alkali solutions [KIR83] |
| Мерк | 13,9143 |
| Пределы воздействия | ACGIH: TWA 0.5 ppm(2.5 mg/m3); Ceiling 2 ppm (Skin) OSHA: TWA 3 ppm NIOSH: IDLH 30 ppm(250 mg/m3); TWA 3 ppm(2.5 mg/m3); Ceiling 6 ppm(5 mg/m3) |
| Стабильность | Stable. Powder is very flamable. |
| ИнЧИКей | GUVRBAGPIYLISA-UHFFFAOYSA-N |
| LogP | -1 at 20℃ |
| Справочник по базе данных CAS | 7440-25-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 6424HBN274 |
| Система регистрации веществ EPA | Tantalum (7440-25-7) |
| UNSPSC Code | 41122203 |
| NACRES | NA.24 |
| Коды опасности | F,Xi,Xn,C | |||||||||
| Заявления о рисках | 11-36/37/38-20/21/22-40-34-36/38 | |||||||||
| Заявления о безопасности | 16-26-33-36/37/39-36-45-27-36/37 | |||||||||
| РИДАДР | UN 3089 4.1/PG 2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3, STEL: 10 mg/m3 | |||||||||
| WGK Германия | - | |||||||||
| RTECS | WW5505000 | |||||||||
| Температура самовоспламенения | 572 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 8103909000 | |||||||||
| Банк данных об опасных веществах | 7440-25-7(Hazardous Substances Data) | |||||||||
| ИДЛА | 2,500 mg Ta/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H228:Воспламеняющееся твердое вещество.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P370+P378:При пожаре тушить сухим песком, сухим химическим порошком или спиртостойкой пеной.
Тантал химические свойства, назначение, производство
Химические свойства
Tantalum is a refractory metal in Group V-B of the periodic table. The pure metal is ductile, steel-blue to gray solid or black, odorless powder.Физические свойства
Tantalum has properties similar to niobium and vanadium above it in group 5. It is a veryhard and heavy metal with a bluish color when in its rough state, but if polished, it has a silveryshine. It is ductile, meaning it can be drawn into fine wires, and also malleable, meaningit can be hammered and worked into shapes. Thin strips and wires of tantalum will ignite inair if exposed to a flame.Tantalum’s melting point is 2,996°C, which is almost as high as tungsten and rhenium. Itboiling point is 5,425°C, and its density is 19.3 g/cm3.
Изотопы
There are 49 isotopes of tantalum. Only the isotope Ta-181 is stable andaccounts for 99.988% of the total mass of the element on Earth. Just 0.012% of the element’s mass is contributed by Ta-180, which has a half-life of 1.2×10+15 years and isthus considered naturally stable. The remaining 47 isotopes are all artificially producedin nuclear reactions or particle accelerators and have half-lives ranging from a few microsecondsto few days to about two years.Происхождение имени
Tantalum was named after Tantalus, who was the father of Niobe, the queen of Thebes, a city in Greek mythology. (Note: The element tantalum was originally confused with the element nobelium.)Вхождение
Tantalum is the 51st most abundant element found on Earth. Although it is found in afree state, it is usually mixed with other minerals and is obtained by heating tantalum potassiumfluoride or by the electrolysis of melted salts of tantalum. Tantalum is mainly obtainedfrom the following ores and minerals: columbite [(Fe, Mn, Mg)(Nb, Ta)2O6]; tantalite [(Fe,Mn)(Ta, Nb)2O6]; and euxenite [(Y, Ca, Er, La, Ce, U, Th)(Nb, Ta, Ti)2O6]. Tantalum’s oresare mined in South America, Thailand, Malaysia, Africa, Spain, and Canada. The UnitedStates has a few small native deposits but imports most of the tantalum it uses.Since tantalum and niobium are so similar chemically, a solvent process must be employedto separate them from the common ores. They are dissolved in a solvent, resulting in 98% pure niobium oxide being extracted during this part of the process. This is followed by 99.5%pure tantalum oxide being extracted in a second solvent process.
Характеристики
Tantalum is almost as chemically inert at room temperatures (it has the ability to resistchemical attacks, including hydrofluoric acid) as are platinum and gold. It is often substitutedfor the more expensive metal platinum, and its inertness makes it suitable for constructingdental and surgical instruments and artificial joints in the human body.Использование
In pen points; analytical weights; apparatus and instruments for chemical, surgical, and dental use instead of platinum, in tantalum capacitors (a type of electrolytic condenser, trademarked "Tantalytic").Методы производства
It was identi?ed that tantalum minerals exists in over 70 differentchemicalcompositions.Thoseofgreatesteconomic importance are tantalite, microlite, and wodginite; however, it is common practice to name any tantalum-containing mineral concentrate as “tantalite”. Tantalum resources are widespread, with the most important known resources being found in Brazil and Australia. In mid-2008, the main mining operations were in Australia, Brazil,Canada,Mozambique,andEthiopiaandinmid-2009, in Brazil, Ethiopia, and China, with additional quantities originating in central Africa, Russia, and Southeast Asia. There is continued interest in exploration of this element in other countries, primarily in Egypt, Canada, Mozambique, and Saudi Arabia.The major world mine producers of tantalum in 2010 were Brazil (180 tons), Mozambique (110 tons), Rwanda (100 tons), and Australia (80 tons). Other countries produced around 170 tons, so the total world production of tantalum was approximately 670 tons. The major producers of tantalum mineral concentrates are Australia, Brazil, and Canada.
Определение
A silvery transition element. It is strong, highly resistant to corrosion, and is easily worked. Tantalum is used in turbine blades and cutting tools and in surgical and dental work. Symbol: Ta; m.p. 2996°C; b.p. 5425 ± 100°C; r.d. 16.654 (20°C); p.n. 73; r.a.m. 180.9479.Общее описание
Tantalum dust is a black odorless powder. Mp: 2996°C; bp: approx. 5250°C. Density: 16.65 g cm-3. Insoluble in water. Tantalum oxide dust is a white, microcrystalline powder Mp: 1800°C. Density: 7.6 g cm-3. Insoluble in water. The mixture is listed as a toxic inhalation hazard by OHSA.Опасность
The dust and powder of tantalum are explosive. Several tantalum compounds are toxic ifinhaled or ingested, but the metal itself is nonpoisonous.Угроза здоровью
Tantalum has a low order of toxicity but has produced transient inflammatory lesions in the lungs of animals. Surgical implantation of tantalum metal products such as plates and screws has not shown any adverse tissue reaction, thus demonstrating its physiological inertness.Профиль безопасности
An inhalation hazard. Some industrial skin injuries from tantalum have been reported. Systemic industrial poisoning, however, is apparently unknown. Questionable carcinogen with experimental tumorigenic data. The dry powder iptes spontaneously in air. Incompatible with bromine trifluoride, fluorine, lead chromate. See also specific tantalum compounds.Канцерогенность
Although Oppenheimer et al., using embedded metal foil technique, have elicited two malignant ?brosarcomas in 50 embeddings of tantalum metal in 25Wistar rats aftera latent period of714days, these results remain a controversial issue. Miller et al. have studied tumorigenic transforming potential of tungsten, iron, nickel, and cobalt with tantalum as a comparison on an immortalized nontumorigenic human osteoblast-like cell line. No tumorigenic activity of Ta was reported, but data are not shown.In the recent study, intramuscularly pellets (1mm 2mm cylinders) of weapons-grade WA were implantedtosimulateshrapnelwounds.Ratswereimplanted with 4 (low dose) or 20 pellets (high dose) of WA. Tantalum (20 pellets) and nickel (20 pellets) served as negative and positive controls, respectively. Rats implanted with tantalum (n=46) did not develop tumors.
Перевозки
UN3089 Metal powders, flammable, n.o.s., Hazard Class: 4.1; Labels: 4.1-Flammable solid.Несовместимости
A flammable solid; the dry powder can ignite spontaneously in air. Incompatible with lead chromate. A strong reducing agent; incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, bromine trifluoride, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Tantalum metal is attacked by hydrogen fluoride, fused alkalis, fuming sulfuric acid.Утилизация отходов
Sanitary landfill if necessary; recover if possible because of economic value. Technology exists for tantalum recovery from spent catalysts, for example.Тантал запасные части и сырье
запасной предмет
Тантал поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 15369953316 +8615369953316 |
China | 2123 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| 02120970332; +8613524231522 |
China | 2904 | 58 | |
| China | 12341 | 58 | ||
| United States | 24072 | 58 | ||
| +8617732866630 | China | 18147 | 58 | |
| +undefined-86-1523780-4566 +undefined15237804566 |
China | 511 | 58 |