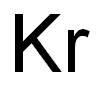
КРИПТОН
- английское имяKRYPTON
- CAS №7439-90-9
- CBNumberCB3305472
- ФормулаKr
- мольный вес83.8
- EINECS231-098-5
- номер MDLMFCD00016158
- файл Mol7439-90-9.mol
химическое свойство
| Температура плавления | -157℃ |
| Температура кипения | bp -153.35° |
| плотность | 908 kg/m3; d0 (101.3 kPa) 3.7493 kg/m3; d (normal bp) 8.6 kg/m3; d (normal bp) 2415 kg/m3; d (triple pt) 2451 kg/m3; d (triple pt) 2826 kg/m3 |
| растворимость | slightly soluble in H2O |
| форма | colorless gas |
| цвет | colorless |
| Растворимость в воде | 59.4mL/1000g H2O (20°C) [KIR78]; Henry’s law constants, k×10?4: 3.685 (70.2°C), 4.017 (175.0°C), 3.761 (175.0°C), 2.392 (252.5°C) [POT78] |
| Мерк | 13,5340 |
| Стабильность | Stable and unreactive; not combustible. |
| FDA UNII | 5I8I620HVX |
| Система регистрации веществ EPA | Krypton (7439-90-9) |
| UNSPSC Code | 12142003 |
| NACRES | NA.22 |
| Заявления о безопасности | 9-38 | |||||||||
| РИДАДР | UN 1950 2.2 | |||||||||
| WGK Германия | 3 | |||||||||
| F | 4.5-31 | |||||||||
| Классификация DOT | 2.2 (Nonflammable gas) | |||||||||
| Класс опасности | 2.2 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
-
оператор предупредительных мер
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
КРИПТОН химические свойства, назначение, производство
Описание
Krypton, Kr, is an elemental, colorless, odorless inert gas. It is noncombustible, nontoxic, and nonreactive; however, it is an asphyxiant gas and will displace oxygen in the air. Krypton 85 is radioactive and has a half-life of 10.3 years. The four-digit UN identification number for krypton is 1056 as a compressed gas and 1970 as a cryogenic liquid. These forms of krypton are not radioactive. Radioactive isotopes of krypton are shipped under radioactive labels and placards as required. Its primary uses are in the activation of phosphors for self-luminous markers, detecting leaks, and in medicine to trace blood flow.Химические свойства
colourless gasФизические свойства
Krypton is a rather dense, tasteless, colorless, odorless gas. Its critical temperature isbetween that of oxygen and carbon dioxide. It is extracted during fractional distillation ofliquid oxygen at a temperature of about –63.8°C. At one time it was thought that krypton, aswell as the other noble gases, were completely inert. However, in 1967 scientists were able tocombine fluorine with krypton at low temperatures to form the compound krypton difluoride(KrF2). In this case krypton has a valence of 2.Krypton’s melting point is –156.6°C, its boiling point is –152.30°C, and its density is0.003733g/cm3.
Изотопы
There are a total of 37 isotopes of krypton. Six of these are stable: Kr-78, Kr-80,Kr-82, Kr-83, Kr-84, and Kr-86. The isotope Kr-78 has such a long half-life (0.9×10+20years) that it is considered stable even though it contributes only 0.35% to the naturalkrypton in the Earth’s atmosphere. All the others are radioactive, man-made by-productsof nuclear power plants and radioactive isotopes with half-lives ranging from 107 nanosecondsto 2.29×10+15 years.Происхождение имени
The name “krypton” is derived from the Greek word kryptos, meaning “hidden.”Вхождение
Krypton is the 81st most abundant element on Earth and ranks seventh in abundance ofthe gases that make up Earth’s atmosphere. It ranks just above methane (CH4) in abundancein the atmosphere. Krypton is expensive to produce and thus has limited use. The gas is capturedcommercially by fractional distillation of liquid air. Krypton shows up as an impurity inthe residue. Along with some other gases, it is removed by filtering through activated charcoaland titanium.There are traces of krypton in some minerals and meteorites. Krypton is found beyondEarth in space.
Характеристики
Krypton is the fourth element in group 18 (VIIIA), which is also known as group 0 becausethe elements is this group were thought to have a zero oxidation point. Krypton has many ofthe chemical properties and characteristics of some of the other noble gases.The fragile compounds formed by noble gases at low temperatures, such as KrF2, are calledclathrates.
Использование
Krypton is expensive to produce, which limits its use as an inert gas. It is used in a mixturewith argon to fill incandescent light bulbs, fluorescent lamps, lasers, and high-speed photographylamps. Radioactive Kr-85 is used as a source of radiation to measure the thickness ofindustrial materials. It is also used to test for “leakage” of scientific instruments.Since 1960 the wavelength of the spectral lines of the krypton-86 isotope has been usedas the standard for the length of the meter. One meter is now defined as 1,650,762.73 wavelengthsof the reddish-orange spectral line of the Kr-86 isotope.
Определение
A colorless odorless monatomic element of the rare-gas group, known to form unstable compounds with fluorine. It occurs in minute quantities (0.001% by volume) in air. Krypton is used in fluorescent lights. Symbol: Kr; m.p. –156.55°C; b.p. –152.3°C; d. 3.749 (0°C) kg m–3; p.n. 36; r.a.m. 83.80.Методы производства
Most krypton produced in commercial scale comes from air. Krypton and other inert gases are obtained from air by a distillation-liquefaction process. Different types of air-separation plants varying in design are known for commercial production of nitrogen, oxygen, and inert gases (See Helium).Krypton also may be recovered from spent fuel rods of nuclear power plants. It is produced, along with xenon, in fission of uranium and plutonium. This process, however, is not a major source of krypton, and the recovered gas also contains radioactive Kr-85 isotope.
.
Общее описание
Krypton, refrigerated liquid, is a colorless, odorless gas. KRYPTON is shipped as a liquid under its own vapor pressure. Contact with the liquid may cause frostbite to unprotected skin. KRYPTON can asphyxiate by displacement of air. Exposure of the container to prolonged heat or fire may cause KRYPTON to rupture violently and rocket.Профиль реактивности
These substances undergo no chemical reactions under any known circumstances. They are nonflammable, noncombustible and nontoxic. They can asphyxiate. Contact of very cold liquefied gas with water may result in vigorous or violent boiling of the product and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, there is the possibility that a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquid gas contacts water in a closed container [Handling Chemicals Safely 1980].Опасность
Being an inert gas, krypton is nontoxic. However, the man-made radioisotopes of kryptoncan cause radiation poisoning.Угроза здоровью
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite.Пожароопасность
Non-flammable gases. Containers may explode when heated. Ruptured cylinders may rocket.Промышленное использование
Krypton, which occurs in the air to the extentof 1 part in 1 million, is a heavy gas used as afiller for fluorescent lamps to decrease filamentevaporation and heat loss and to permit highertemperatures in the lamp. Krypton-85, obtainedfrom atomic reactions, is a beta-ray emitter usedin luminous paints for activating phosphors andalso as a source of radiation.КРИПТОН запасные части и сырье
сырьё
1of2
запасной предмет
КРИПТОН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| 18824865657 | China | 509 | 58 | |
| 0510-051082803581 15903302207 |
China | 229 | 58 | |
| 18098161577 | China | 168 | 58 | |
| 159-0619-7626 4001882517 |
China | 528 | 58 | |
| 021-61415566 800-8193336 |
China | 51456 | 80 |