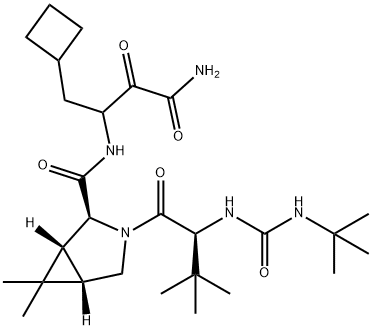
Боцепревир
- английское имяBoceprevir
- CAS №394730-60-0
- CBNumberCB32491465
- ФормулаC27H45N5O5
- мольный вес519.68
- EINECS800-043-2
- номер MDLMFCD22208555
- файл Mol394730-60-0.mol
| Температура плавления | >107°C (dec.) |
| плотность | 1.162 |
| температура хранения | -20°C |
| растворимость | Soluble in DMSO (up to 15 mg/ml with warming) |
| пка | 12.82±0.40(Predicted) |
| форма | solid |
| цвет | White or off-white |
| Стабильность | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| FDA UNII | 89BT58KELH |
| Словарь наркотиков NCI | boceprevir |
| Код УВД | J05AP03 |
| UNSPSC Code | 12352107 |
| NACRES | NA.77 |
| кода HS | 2933599590 | |||||||||
| Банк данных об опасных веществах | 394730-60-0(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H361f:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Боцепревир химические свойства, назначение, производство
Описание
In May 2011, the U.S. FDA approved boceprevir (SCH-503034), to be given in combination with peginterferon alfa plus ribavirin, for the treatment of patients with chronic hepatitis C genotype 1 viral infection. Boceprevir and telaprevir are the first hepatitis C virus (HCV) protease inhibitors to be approved for the treatment of HCV infection. Boceprevir is an inhibitor of HCV NS3-4A protease, an essential enzyme required by HCV for posttranslational processing of viral proteins into their mature forms. Boceprevir binds covalently, but reversibly, to the active site serine by addition of the hydroxyl group to the keto-amide functionality. Boceprevir inhibits HCV NS3-4A protease with a Ki of 14 nM. In cell culture, the EC50 of boceprevir was 200 nM for an HCV replicon constructed from genotype 1b. Boceprevir was two-to threefold less potent against HCV replicon from genotypes 1a, 2, and 3. The potency of boceprevir decreased threefold in the presence of human serum. Boceprevir was discovered through a series of systematic truncations and modifications of a keto-amide undecapeptide lead molecule.Boceprevir is synthesized by coupling of 3-amino-4- cyclobutyl-2-hydroxybutyramide or the related oxobutyramide with a cyclopropyl-pyrrolidine carboxylic acid intermediate. The pyrrolidine derivative can be prepared via cyclopropanation of a bicyclic lactam derivative or by conversion of 3,3-dimethylcyclopropane-1,2-dicarboxylic acid to the pyrrolidine in a multistep route. Boceprevir is a 1:1 mixture of diastereomers at the readily epimerizable position a to the keto group.
Химические свойства
Off-White to Pale Yellow SolidИспользование
An NS3 serine protease inhibitor of hepatitis C virus, for the treatment of HCV infection. It is a COVID19-related research product.Определение
ChEBI: A synthetic tripeptide consisting of N-(tert-butylcarbamoyl)-3-methyl-L-valyl, a cyclopropyl-fused prolyl and 3-amino-4-cyclobutyl-2-oxobutanamide residues joined in sequence. Used for treatment of chronic h patitis C virus genotype 1 infection.Клиническое использование
Boceprevir is an oral inhibitor of HCV NS3/4A protease for the treatment of the chronic hepatitis C genotype infection. It is approved as combination therapy with Peg-IFN-alpha and ribavarin to treat adult patients with compensated liver diseasewhoare either treatment naive or who have experienced prior failed therapy with interferon and ribavarin. Boceprevir was initially discovered by Schering-Plough and developed and marketed by Merck & Co. since its acquisition of Schering-Plough in 2009. Several publications have highlighted the discovery of this drug, which evolved from a potent initial undecapeptide lead structure to boceprevir (VII) as a drug candidate with potent activity and desirable PK properties.Боцепревир запасные части и сырье
сырьё
- Cyclobutanebutanoic acid, β-[[(1,1-dimethylethoxy)carbonyl]amino]-α-hydroxy-
- N-МЕТИЛМОРФОЛИНА ГИДРОХЛОРИД
- ТРЕТ-БУТИЛ-1-ЦИАНО-3-ЦИКЛОБУТИЛ-1-ГИДРОКСИПРОПАН-2-ИЛКАРБАМАТ
- (бромметил)циклобутан
- (1R,2S,5S)-3-[(S)-2-(3-TERT-BUTYL-UREIDO)-3,3-DIMETHYL-BUTYRYL]-6,6-DIMETHYL-3-AZA-BICYCLO[3.1.0]HEXANE-2-CARBOXYLIC ACID
- ВОС-DL-ЦИКЛОБУТИЛАЛАНИН
- 1025799-39-6
- Этил 2-амино-3-циклобутилпропаноат
- циклобутанбутанамид, β-амино-α-оксо-, гидрохлорид (1:1)
- 3-Azabicyclo[3.1.0]hexane-2-carboxaMide, N-[3-aMino-1-(cyclobutylMethyl)-2-hydroxy-3-oxopropyl]-3-[(2S)-2-[[[(1,1 -diMethylethyl)aMino]carbonyl]aMino]-3,3-diMethyl-1-oxobutyl]-6,6-diMe thyl-, (1R,2S,5S)-
1of3
Боцепревир поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21622 | 55 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29855 | 58 | |
| 0086-13720134139 | CHINA | 965 | 58 | |
| +8618523575427 | China | 49730 | 58 | |
| +1-781-999-5354; +17819995354 |
United States | 32376 | 58 | |
| +86-02584696168 15380713688 |
CHINA | 865 | 58 | |
| +8617735180244 | CHINA | 4017 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3134 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 21550 | 58 |