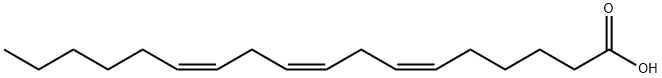
gamma-Linolenic acid
- русский язык имя
- английское имяgamma-Linolenic acid
- CAS №506-26-3
- CBNumberCB3238150
- ФормулаC18H30O2
- мольный вес278.43
- номер MDLMFCD00065718
- файл Mol506-26-3.mol
| Температура плавления | -12~-11℃ |
| Температура кипения | 379.5±11.0 °C(Predicted) |
| плотность | 0.92 |
| показатель преломления | n |
| Fp | 110 °C |
| температура хранения | -20°C |
| растворимость | Chloroform (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| форма | liquid |
| пка | 4.74±0.10(Predicted) |
| цвет | Colourless |
| Биологические источники | synthetic |
| Мерк | 14,5507 |
| БРН | 1712253 |
| Стабильность | Light Sensitive |
| ИнЧИКей | VZCCETWTMQHEPK-QNEBEIHSSA-N |
| LogP | 6.570 (est) |
| Справочник по базе данных CAS | 506-26-3(CAS DataBase Reference) |
| FDA UNII | 78YC2MAX4O |
| Справочник по химии NIST | Gamolenic acid(506-26-3) |
| Код УВД | D11AX02,D11AX52 |
| UNSPSC Code | 85151701 |
| NACRES | NA.25 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| WGK Германия | 3 | |||||||||
| F | 8-10-23 | |||||||||
| кода HS | 29161500 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
gamma-Linolenic acid химические свойства, назначение, производство
Описание
γ-Linolenic acid (gamma-linolenic acid or GLA, INN and USAN gamolenic acid) is a fatty acid found primarily in vegetable oils. It is sold as a dietary supplement for treating problems with inflammation and auto-immune diseases, although its efficacy is disputed.Химические свойства
GLA is categorized as an n?6 (also called ω?6 or omega-6) fatty acid, meaning that the first double bond on the methyl end (designated with n or ω) is the sixth bond. In physiological literature, GLA is designated as 18:3 (n?6). GLA is a carboxylic acid with an 18-carbon chain and three cis double bonds. It is an isomer of α-linolenic acid, which is a polyunsaturated n?3 (omega-3) fatty acid, found in rapeseed canola oil, soy beans, walnuts, flax seed (linseed oil), perilla, chia, and hemp seed.Вхождение
GLA is obtained from vegetable oils such as GLA safflower oil (Carthamus tinctorius), evening primrose (Oenothera biennis) oil, blackcurrant seed oil, borage oil, and hemp seed oil. GLA is also found in edible hemp seeds, oats, barley, and spirulina. Each contains varying amounts of the fatty acid, with GLA safflower oil at 40% GLA being a novel concentrated form. This is a new genetically modified oil and has been available in commercial quantities since 2011. It should be noted that conventional safflower oils have zero GLA. Borage oil ranges from 15 % to 20 % and evening primrose oil ranges from 8 % to 10 % GLA.The human body produces GLA from linoleic acid (LA). This reaction is catalyzed by Δ6 - desaturase (D6D), an enzyme that allows the creation of a double bond on the sixth carbon counting from the carboxyl terminus. LA is consumed sufficiently in most diets, from such abundant sources as cooking oils and meats. However, a lack of GLA can occur when there is a reduction of the efficiency of the D6D conversion (for instance, as people grow older or when there are specific dietary deficiencies) or in disease states wherein there is excessive consumption of GLA metabolites.
История
GLA was first isolated from the seed oil of evening primrose. This herbal plant was grown by Native Americans to treat swelling in the body. In the 17th century, it was introduced to Europe and became a popular folk remedy, earning the name king's cure-all. in 1919, Heiduschka and Lüft extracted the oil from evening primrose seeds and described an unusual linolenic acid, which they name γ-. Later, the exact chemical structure was characterized by Riley.Although there are α- and γ- forms of linolenic acid, there is no β- form. One was once identified, but it turned out to be an artifact of the original analytical process.
Использование
γ-Linolenic Acid is a fatty acid found mainly in vegetable oil. Studies suggest that γ-Linolenic Acid may have immune regulating and anti-inflammatory effects. γ-Linolenic Acid has also been used in the treatment of atopic eczeme although its efficacy isОпределение
ChEBI: A C18, omega-6 acid fatty acid comprising a linolenic acid having cis- double bonds at positions 6, 9 and 12.gamma-Linolenic acid запасные части и сырье
gamma-Linolenic acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8618629063126 | China | 435 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86-28-82633860; +8618080483897 |
China | 3772 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| +86-15200345168 +86-15200345168 |
CHINA | 151 | 58 |