GALLIUM NITRATE
- русский язык имя
- английское имяGALLIUM NITRATE
- CAS №13494-90-1
- CBNumberCB3209552
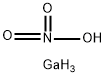
- ФормулаGaH4NO3
- мольный вес135.76
- EINECS236-815-5
- номер MDLMFCD00011016
- файл Mol13494-90-1.mol
| Температура плавления | 110°C (dec.) |
| растворимость | soluble in H2O, ethanol, ethyl ether |
| форма | Liquid |
| цвет | white crystals, crystalline powder |
| Растворимость в воде | It is soluble in water. |
| Пределы воздействия | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| FDA UNII | Y2V2R4W9TQ |
| Словарь наркотиков NCI | Ganite |
| Система регистрации веществ EPA | Gallium nitrate (13494-90-1) |
| UNSPSC Code | 12352200 |
| Заявления о рисках | 8-36/37/38 | |||||||||
| Заявления о безопасности | 17-26-36/37/39 | |||||||||
| РИДАДР | 1477 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 5.1 | |||||||||
| Группа упаковки | III | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H272:Окислитель; может усилить возгорание.
-
оператор предупредительных мер
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P405:Хранить в недоступном для посторонних месте.
GALLIUM NITRATE химические свойства, назначение, производство
Описание
Gallium nitrate, initially developed as an anticancer agent, was introduced by Fujisawa as an orphan drug for the treatment of cancer-related hypercalcemia and bone metastases that do not respond to adequate hydration. The compound acts specifically on bone by inhibiting calcium resorption and also possibly by stimulating bone formation. Compared with calcitonin and etidronate, gallium nitrate is more potent and substantially longer acting. Other potential uses could be in the treatment of osteoporosis and Paget’s disease.Химические свойства
The structural formula of gallium nitrate is Ga(NO3)3. Gallium nitrate is an anhydrate salt that is very soluble in water and soluble in 95% ethanol. It is stable in commonly used intravenous fluids for 14 days at room temperature and at 5 ℃, and physically compatible for injection with selected drug products, with the exception of diazepam injection.This chemical is an oxidizer, and is probably combustibleИспользование
Gallium is the second metal ion with clinical activity used in cancer treatment. Gallium nitrate has demonstrated antitumor activity in a variety of murine tumor models, including Walker 256 carcinosarcoma, fibrosarcoma M-89, leukemia K-1964, adenocarcinoma 755, mammary carcinoma YMC, reticulum cell sarcoma A-RCS, lymphoma P1798, and osteosarcoma 124F. It was, however, ineffective in ascites, leukemias, plasma cell tumors, or Ehrlich carcinoma. In phase II evaluation studies, gallium nitrate has shown antitumor activity in patients with either refractory lymphomas or small-cell lung carcinomas or bladder cancer, with total objective response rates of 28% and 11%, respectively. This drug has displayed its strongest antineoplastic activity in the treatment of non-Hodgkin’s lymphoma and bladder cancer.In addition, according to two phase III comparative trials,thanks to an inhibitory effect on calcium reabsorption from bone, gallium nitrate is superior to alternative therapies in the treatment of hypercalcemia associated with malignancy and related disease states. Based on its clinical efficacy, gallium nitrate (Ganite ) was approved by the Food and Drug Administration (FDA) for the treatment of cancerassociated hypercalcemia.
Apart from its ability to control hypercalcemia, gallium nitrate has been shown to inhibit bone turnover and decrease osteolysis in patients with multiple myeloma and in patients with bone metastases from a variety of different cancers.
Общее описание
White crystals.Реакции воздуха и воды
Deliquescent. Water soluble.Профиль реактивности
Oxidizing agents, such as GALLIUM NITRATE, can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds, in general, have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates.Пожароопасность
Flash point data for GALLIUM NITRATE are not available, but GALLIUM NITRATE is probably combustible.Фармацевтические приложения
In clinical trials, gallium nitrate has proved to be highly active as an antitumour agent especially against non-Hodgkin’s lymphoma and bladder cancer. The cytotoxic activity of gallium nitrate has been demonstrated as single agent and as part of combination therapy, for example, together with fluorouracil. Gallium nitrate shows a relatively low toxicity and does not produce myelosuppression, which is a significant advantage over other traditional anticancer agents. Furthermore, it does not appear to show any cross-resistance with conventional chemotherapeutic agents.These studies have also shown that gallium nitrate is able to decrease serum calcium levels in patients with tumour-induced hypercalcaemia. Subsequently, several studies have been carried out comparing traditional bisphosphonate drugs with gallium nitrate in their ability to decrease the calcium levels that are elevated as a result of cancer. Based on the clinical efficacy, gallium nitrate injections (GaniteTM) was granted approval by the FDA for the treatment of cancer-associated hypercalcaemia. Gallium nitrate is also believed to inhibit the bone turnover and therefore to decrease osteolysis, the active reabsorption of bone material, in patients with bone metastasis secondary to other cancers.
Канцерогенность
Studies on the antitumor activity of gallium nitrate have shown that it is particularly active against solid tumors. It has demonstrated antitumor activity in a variety of murine tumor models, including Walker carcinosarcoma 256, fibrosarcoma M-89, leukemia K-1964, adenocarcinoma 755, mammary carcinoma YMC, reticulum cell sarcoma A-RCS, lymphoma P1798, and osteosarcoma 124F.GALLIUM NITRATE запасные части и сырье
сырьё
GALLIUM NITRATE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8617531153977 | China | 5855 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-852-30606658 | China | 24727 | 58 | |
| +86-057181025280; +8617767106207 |
China | 49734 | 58 | |
| +8618950047208 | China | 43416 | 58 |
GALLIUM NITRATE Обзор)
- Неодимовый (III) гексагидрат нитрата
- Лантан (III) гексагидрата нитрата
- Лантана (III) гидрата нитрата
- Никель (II) гексагидрата нитрата
- Гольмиевый (III) нитрат пентагидра
- Тербий(III) ацетат гидрат
- Тулий(III) ацетат гидрат
- Рубидий
- Германий
- Празеодим (III) гексагидрата нитрата
1of4