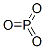
Phosphorus trioxide
- русский язык имя
- английское имяPhosphorus trioxide
- CAS №1314-24-5
- CBNumberCB2852146
- ФормулаH4O5P2
- мольный вес145.976282
- номер MDLMFCD09970509
- файл Mol1314-24-5.mol
химическое свойство
| Температура плавления | 23.8° | ||||||||||||||
| Температура кипения | bp 173.1° in nitrogen atm | ||||||||||||||
| плотность | d421 2.135 | ||||||||||||||
| растворимость | reacts with H2O | ||||||||||||||
| форма | colorless monoclinic crystals or liquid | ||||||||||||||
| цвет | colorless monoclinic crystals, crystalline or liquid | ||||||||||||||
| Растворимость в воде | slowly forms H3PO3 in cold H2O; reacts violently in hot H2O forming red P, PH3, and H3PO4 [MER06] | ||||||||||||||
| crystal system | Monoclinic | ||||||||||||||
| Space group | P21/m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| FDA UNII | 0LTR52K7HK | ||||||||||||||
| Система регистрации веществ EPA | Phosphorus trioxide (1314-24-5) |
Phosphorus trioxide химические свойства, назначение, производство
Химические свойства
transparent crystal(s); monoclinic or colorless liquid; disproportionates to red P and P2O4if heated above 210°C [MER06]Общее описание
White crystalline solid or a liquid (melting point 24°C). Density 2.14 g / cm3. Toxic and corrosive. May severely irritate skin and eyes. Used to make other chemicals.Реакции воздуха и воды
Fumes in air. Reacts slowly with cold water to give phosphorous acid. Reacts vigorously with hot water to generate red phosphorus, phosphine (highly toxic and flammable) and phosphoric acid [Merck 11th ed. 1989].Профиль реактивности
Phosphorus trioxide reacts exothermically with bases. Melted Phosphorus trioxide readily ignites in air. Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60°C [J. Am. Chem. Soc. 125:347. 1924]. Incompatible with ammonia, disulfur dichloride, halogens, oxygen, phosphorus pentachloride, sulfur, sulfuric acid, water [Bretherick, 5th ed., 1995, p. 1778].Угроза здоровью
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.Phosphorus trioxide запасные части и сырье
запасной предмет
Phosphorus trioxide поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 021-5983532 15201998618 |
China | 4616 | 58 | |
| 0751-2815987 13927875076 |
China | 9968 | 58 | |
| 400-666-9280 18026564465 |
China | 8011 | 58 | |
| 029-029-63373950 15353716720 |
China | 10002 | 58 |