Teriparatide acetate
- русский язык имя
- английское имяTeriparatide acetate
- CAS №52232-67-4
- CBNumberCB2284468
- ФормулаC172H278N52O47S2
- мольный вес3890.49792
- EINECS640-978-1
- номер MDLMFCD00149013
- файл Mol52232-67-4.mol
| Температура плавления | >205oC (dec.) |
| RTECS | SQ7770000 |
| температура хранения | −20°C |
| растворимость | DMSO (Slightly), Water (Slightly) |
| форма | powder |
| цвет | White to Off-White |
| Биологические источники | human |
| Растворимость в воде | Soluble to 0.40 mg/ml in water |
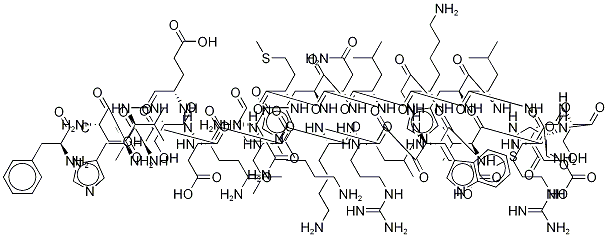
| Последовательность | H-Ser-Val-Ser-Glu-Ile-Gln-Leu-Met-His-Asn-Leu-Gly-Lys-His-Leu-Asn-Ser-Met-Glu-Arg-Val-Glu-Trp-Leu-Arg-Lys-Lys-Leu-Gln-Asp-Val-His-Asn-Phe-OH |
| Стабильность | Hygroscopic |
| Справочник по базе данных CAS | 52232-67-4(CAS DataBase Reference) |
| FDA UNII | 10T9CSU89I |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
| WGK Германия | 3 |
| кода HS | 2937190000 |
| Банк данных об опасных веществах | 52232-67-4(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P370+P378:При пожаре тушить сухим песком, сухим химическим порошком или спиртостойкой пеной.
P403+P235:Хранить в прохладном, хорошо вентилируемом месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Teriparatide acetate химические свойства, назначение, производство
Описание
The anabolic drug teriparatide acetate (TA), known as recombinant human parathyroid hormone 1–34, which directly promotes bone formation by generating new osteocytes, has been introduced as a novel therapeutic agent for osteoporosis. Distinct from antiresorptive drug treatment, patients with osteonecrosis of the jaw showed successful clinical outcomes after weekly administration of TA. In addition, adverse outcomes of long-term bisphosphonate treatment, such as bone fracture, were healed after usage of TA, and better bone mass and mineral density improvements at the lumbar spine and femoral neck were achieved with TA treatment than with bisphosphonate treatment[1].Использование
A fragment of human parathyroid hormone (hPTH) peptide sequence containing the 34 N-terminal residues of hPTH. This fragment was also found to be an agonist at PTH1 and PTH2 receptors.Общее описание
Teriparatide is a recombinantform of parathyroid hormone, which is used for the treatmentof osteoporosis in men and postmenopausal women.The N-terminal region possesses 34 amino acids, which areidentical to the biologically active region of the 84-aminoacid sequence of human parathyroid hormone. It has beenshown to act on osteoblasts to stimulate new bone growthand improve bone density.Механизм действия
Teriparatide acetate is the salt form of teriparatide and has a similar mechanism of action to teriparatide. Teriparatide is a parathyroid hormone (PTH) analogue that regulates calcium and phosphate metabolism in bone and kidney by binding to the PTH type 1 receptor (PTH1R type) via the N-terminal portion. It has the same effect on calcium-phosphorus homeostasis as endogenous PTH (i.e., increases serum calcium and decreases serum phosphorus).Фармакокине?тика
If administered once daily or intermittently, teriparatide preferentially enhances osteoblastic function,and bone formation occurs. Continuous exposure to endogenous PTH may result in poor skeletal composition because of enhanced osteoclast-mediated bone resorption. After 18 months of treatment, lumbar BMD increased up to 12% in postmenopausal women. After 10 months of treatment, 53% of men had an increase of 5% or greater in spine BMD. The risk for developing new vertebral fractures was reduced by 65% after 21 months of treatment, and the number of nonvertebral fragility fractures was reduced by 53%.Клиническое использование
In 2002, the U.S. FDA approved teriparatide for the treatment of postmenopausal osteoporosis in patients who have a high risk of fracture as well as to increase bone mass in men with primary or hypogonadal osteoporosis who have a high risk of fracture. Teriparatide is recombinant human PTH 1-34, the biologically active portion of the endogenously produced preprohormone. Unlike the bisphosphonates, which are classified as bone restorative agents, teriparatide is the first approved bone-forming agent. Bone formation is possible because of the ability of this agent to increase the number of osteoblasts. Although teriparatide enhances the function of both osteoclasts and osteoblasts, the exposure incidence dictates its effect on the skeleton.Побочные эффекты
Temporary increases in serum calcium levels occur following administration of teriparatide. As a result, this agent is contraindicated in patients who are predisposed to hypercalcemia. Some evidence suggests that these elevations in serum calcium levels may cause a patient who is taking digitalis to experience digitalis toxicity (39). Teriparatide should not be prescribed to patients with Paget's disease, children, young adults, women who are pregnant or nursing, and those patients who have received skeletal radiation therapy. Because of an increased incidence of osteosarcoma (malignant bone tumors) observed in rats, teriparatide also carries a black box warningДозировка
Teriparatide acetate requires subcutaneous injection into the thigh or abdominal wall, and the recommended dose is 20 mcg once daily. If symptoms of orthostatic hypotension occur, it is administered while the patient is sitting or lying down. It is not recommended to use the drug for more than 2 years in the lifetime of the patient. Use teriparatide injection with caution in patients receiving digoxin. Transient hypercalcemia may predispose patients to digitalis toxicity.Teriparatide acetate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13865152372 +86-13865152372 |
China | 71 | 58 | ||
| 0551-65326643 18156095617 |
China | 995 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +86-15373193816 +86-15373193816 |
China | 269 | 58 | ||
| +8618092446649 | China | 1143 | 58 | ||
| +86-027-59207850 | China | 5972 | 58 | ||
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | ||
| +86-852-57055271 +8613237129059 |
China | 232 | 58 | ||
| +8613363867578 | China | 151 | 58 | ||
| +8617621705551 | China | 264 | 58 |