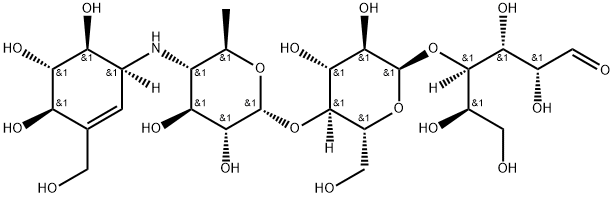
Акарбоза
- английское имяAcarbose
- CAS №56180-94-0
- CBNumberCB2253114
- ФормулаC25H43NO18
- мольный вес645.61
- EINECS260-030-7
- номер MDLMFCD00869592
- файл Mol56180-94-0.mol
химическое свойство
| Температура плавления | 165-170°C |
| альфа | D18 +165° (c = 0.4 in water) |
| Температура кипения | 675.05°C (rough estimate) |
| плотность | 1.4278 (rough estimate) |
| показатель преломления | 1.6000 (estimate) |
| температура хранения | 2-8°C |
| растворимость | Very soluble in water, soluble in methanol, practically insoluble in methylene chloride. |
| пка | 12.39±0.20(Predicted) |
| форма | Solid |
| цвет | White to Off-White |
| Биологические источники | bacterial (Actinoplanes) |
| Растворимость в воде | Soluble in water. |
| Мерк | 14,18 |
| Стабильность | Hygroscopic |
| ИнЧИКей | XUFXOAAUWZOOIT-JMPDRRIHSA-N |
| LogP | -7.935 (est) |
| Справочник по базе данных CAS | 56180-94-0 |
| FDA UNII | T58MSI464G |
| Словарь наркотиков NCI | acarbose |
| Код УВД | A10BF01 |
| UNSPSC Code | 41116107 |
| NACRES | NA.25 |
больше
| Заявления о безопасности | 24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | LZ7153000 | |||||||||
| кода HS | 29400090 | |||||||||
| Банк данных об опасных веществах | 56180-94-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orl-rat: 24 g/kg NIIRDN -,2,1995 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Акарбоза химические свойства, назначение, производство
Описание
Acarbose, a complex oligosaccharide isolated from Actinoplanes, is reportedly useful as an adjuvant therapy in diabetes. By inhibiting alpha-glucosidase, acarbose delays carbohydrate metabolism in the gastrointestinal tract and modulates changes in food induced blood sugar levels.Химические свойства
Off-White SolidИспользование
Acarbose is pseudo-oligosaccharide with a terminal C7-cyclitol patented in 1975 by Bayer. Acarbose is a component of the amylostatin complex produced by a species of Actinoplanes and Streptomyces. Acarbose acts as a potent inhibitor of alpha-glucosidases and saccharases. Since 1990, acarbose has been used therapeutically for the treatment of type 2 diabetes.Определение
ChEBI: Acarbose is a tetrasaccharide derivative consisting of a dideoxy-4-{[4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl C7 cyclitol moiety [called valienol (or valienamine)] linked via nitrogen to isomaltotriose. It has a role as an EC 3.2.1.20 (alpha-glucosidase) inhibitor, an EC 3.2.1.1 (alpha-amylase) inhibitor, a hypoglycemic agent and a geroprotector. It is a conjugate base of an acarbose(1+).Всемирная организация здравоохранения(ВОЗ)
Acarbose, ana-glucosidase inhibitor, is used as an adjunctive therapy in the control of postprandial hyperglycaemia in non-insulin-dependent diabetes mellitus. Use of two or more hypoglycaemic drugs is not recommended.Общее описание
Acarbose is a naturally occurring oligosaccharide,which is obtained from the microorganism Actinoplanes utahensis. It is a white to off-white powder that is soluble in water and has a pKa of 5.1. As one mightexpect, its affinity for α-glucosidase is based on it being apolysaccharide that the enzyme attempts to hydrolyze. Thisallows acarbose to act as a competitive inhibitor, which inturn reduces the intestinal absorption of starch, dextrin, anddissacharides.It is sold as 25-, 50-, and 100-mg tablets (Precose,generics) dosed with the first bite of each meal, up to t.i.d. Acarbose potently inhibits the glucoamylase activity of MGAM α-glucosidases and the sucrase activity of α-glucosidases, where as isomaltase activity is at most moderately inhibited at concentrations in the range of those in theintestinal lumen upon oral dosing, and trehalase and lactaseare not significantly inhibited. Some inhibition of pancreaticα-amylases may also contribute to the clinical effects.
https://www.sciencedirect.com
Клиническое использование
Based on the structure of acarbose, it should come as nosurprise that little intact acarbose reaches the systemic circulation;instead, acarbose is extensively biotransformed by the actionof microbes and digestive enzymes in the gut. Only about35% of the radioactivity in a dose of 14C-labeled acarbose administeredorally to men was excreted in the urine, appearingas several metabolites, some of which are phase II conversionproducts of 4-methylpyrogallol (O-methyl, O-sulfate, orO-glucuronide conjugates); the methylpyrogallol fragmentarises from the terminal valienamine pseudosugar. That thesebiotransformation products are mostly formed in the gut isshown by the fact that nearly 90% of an intravenously administereddose of acarbose is excreted intact in urine.Профиль безопасности
Low toxicity by ingestion,subcutaneous, and intravenous routes. Human systemiceffects: liver function impaired. When heated todecomposition it emits toxic vapors of NOx.Акарбоза поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-021-56795779 +8613651600618 |
China | 1227 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| 18758118018 | China | 255 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8618092446649 | China | 1143 | 58 |