Bladex
- русский язык имя
- английское имяBladex
- CAS №21725-46-2
- CBNumberCB2229243
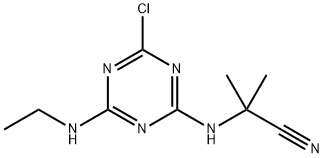
- ФормулаC9H13ClN6
- мольный вес240.69
- EINECS244-544-9
- номер MDLMFCD00055514
- файл Mol21725-46-2.mol
| Температура плавления | 167°C |
| Температура кипения | 383.12°C (rough estimate) |
| плотность | 1.2870 (rough estimate) |
| показатель преломления | 1.6000 (estimate) |
| Fp | 100 °C |
| температура хранения | APPROX 4°C |
| растворимость | DMSO: 100 mg/mL (415.47 mM) |
| форма | Granular Powder |
| пка | 1.46±0.41(Predicted) |
| цвет | Off-white |
| Растворимость в воде | 0.0171 g/100 mL |
| Мерк | 13,2714 |
| БРН | 615509 |
| Справочник по базе данных CAS | 21725-46-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5 |
| FDA UNII | W34C4P18WD |
| Предложение 65 Список | Cyanazine |
| Справочник по химии NIST | Cyanazine(21725-46-2) |
| Система регистрации веществ EPA | Cyanazine (21725-46-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn;N,N,Xn,T,F |
| Заявления о рисках | 22-50/53-39/23/24/25-23/24/25-36-20/21/22-11-52/53 |
| Заявления о безопасности | 37-60-61-45-36/37-36-26-16 |
| РИДАДР | UN 2588/2811 |
| WGK Германия | 3 |
| RTECS | UG1490000 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Банк данных об опасных веществах | 21725-46-2(Hazardous Substances Data) |
| Токсичность | LD50 in rats, mice (mg/kg): 182, 380 orally (Chapman) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P391:Ликвидировать просыпания/проливы/утечки.
P405:Хранить в недоступном для посторонних месте.
Bladex химические свойства, назначение, производство
Химические свойства
Cyanazine is an off-white to tan crystalline solid. Molecular . Hazard identification (based on NFPA-704 M Rating System): Health 2, flammability 1, reactivity 0. Soluble in water. Physical properties may be altered by car- rier solvents used in commercial formulations.Использование
Selective pre-emergence herbicide.Общее описание
Colorless crystals. Non corrosive when dry. Used as a selective systemic herbicide.Реакции воздуха и воды
Stable at pHs between 5.0 and 9.0, but is hydrolyzed by strong acids and bases.Профиль реактивности
A triazine derivative.Опасность
Very toxic.Сельскохозяйственное использование
Herbicide: On August 2, 1995, the U.S. EPA announced a voluntary phase-out of the use of cyanazine. On January 6, 2000, the U.S. EPA announced the cancellation of all cyanazine registrations. It had been A U.S. EPA restricted Use Pesticide (RUP) because of its teratogenicity and because it has been found in groundwater. Not approved for use in EU countries. Cyanazine is used as a pre-and post-emergent herbicide to control annual grasses and broadleaf weeds. It is used mostly on corn, some on cotton, and less than 1% on grain sorghum and wheat fallow. The compound is formulated as a wettable powder, a flowable suspension, or as granules. In California, major usages are on cotton, corn and corn for forage, almonds, and uncultivated agricultural areas. In 1995, cyanazine was the fourth most widely used synthetic pesticide in the U.S. Cyanazine, atrazine, and simazine are collectively referred to as the triazines and may be alternatively used for each other on corn and in some other situations.Торговое название
BLADEX®[C]; BLADEX® 80WP[C]; BULLET®; CYCLE®; CY-PRO®[C]; CYNEX® 41[C]; DW 3418®; EXTRAZINE®[C]; FORTROL®; MATCH®; PAYZE®; SD 15418®; WL 19805®Возможный контакт
A potential danger to those involved in the manufacture, formulation, and application of this her- bicide used for preemergence or postemergence weed con- trol in field corn.Канцерогенность
No increase in tumor incidence was noted in rats fed up to 50 ppm of cyanazine for 2 years. The same was true for mice given up to 1000 ppm in their diets for 2 years.Экологическая судьба
Soil. In sandy loam soils (foc = 0.01), the half-life is 12–15 days. However, in silt loam (foc = 0.028) and clay loam (foc = 0.03) soils, the reported half-life is 20–25 days (Humburg et al., 1989). The half-life of cyanazine is longer in alkaline soils (pH >7.5) than in acidic soils (pH <5.5) (Humburg et al., 1989)Groundwater. According to the U.S. EPA (1986) cyanazine has a high potential to leach to groundwater. Cyanazine amide was identified as a metabolite in groundwater in corn fields (Muir and Baker, 1976)
Plant. In plants, cyanazine is degraded by elimination of the ethyl group, hydration of the cyano group and the removal and replacement of the chlorine atom by a hydroxyl group (Humburg et al., 1989).
Chemical/Physical. In laboratory tests, the nitrile group was hydrolyzed to the corresponding carboxylic acid. The rate of hydrolysis is faster under higher temperatures and low pHs (Grayson, 1980). The chlorine atom may be replaced by a hydroxyl group forming 2((4-hydroxy-6-(ethylamino)-1,3,5-triazin-2-yl)amino)-2-methylpropanenitrile (Hartley and Kidd, 1987)
Перевозки
UN2763 Triazine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3439 Nitriles, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1- Poisonous materials, Technical Name Required.Несовместимости
Cyanazine decomposes in heat producing very toxic fumes and gases of hydrogen cyanide, hydrogen chloride; ethyl chloride; ammonia; acetone; and ethylene. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cya- nide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are Soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids .Attacksmetals in the presence of heat and moisture.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinera- tor equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recom- mendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following pack- age label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.Bladex запасные части и сырье
Bladex поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +8618530059196 | China | 11805 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 |