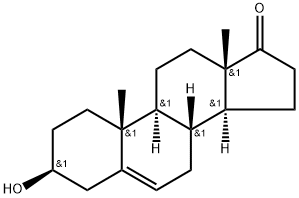
Дегидроэпиандростерон
- английское имяDehydroepiandrosterone
- CAS №53-43-0
- CBNumberCB1475670
- ФормулаC19H28O2
- мольный вес288.43
- EINECS200-175-5
- номер MDLMFCD00003613
- файл Mol53-43-0.mol
| Температура плавления | 149-151 °C(lit.) |
| альфа | 12 º (c=2, ethanol 96% 25 ºC) |
| Температура кипения | 370.65°C (rough estimate) |
| плотность | 1.0484 (rough estimate) |
| показатель преломления | 1.4709 (estimate) |
| Fp | 9℃ |
| температура хранения | Hormones |
| растворимость | insoluble in H2O; ≥13.7 mg/mL in DMSO; ≥58.6 mg/mL in ETOH |
| пка | 15.02±0.60(Predicted) |
| форма | Fine Crystalline Powder |
| цвет | White |
| Биологические источники | synthetic (organic) |
| оптическая активность | +10.918 (c 1, ethanol) |
| Растворимость в воде | 21.8mg/L(23.5 ºC) |
| Мерк | 2871 |
| БРН | 2058110 |
| ИнЧИКей | FMGSKLZLMKYGDP-USOAJAOKSA-N |
| LogP | 3.230 |
| Справочник по базе данных CAS | 53-43-0 |
| Рейтинг продуктов питания EWG | 1-3 |
| Словарь онкологических терминов NCI | dehydroepiandrosterone; DHEA |
| FDA UNII | 459AG36T1B |
| Словарь наркотиков NCI | prasterone |
| Код УВД | A14AA07,G03XX01 |
| Справочник по химии NIST | 5-Androstene-3«beta»-ol-17-one(53-43-0) |
| Система регистрации веществ EPA | Prasterone (53-43-0) |
| UNSPSC Code | 51111800 |
| NACRES | NA.77 |
| Коды опасности | Xi,T,F |
| Заявления о рисках | 36/37/38-39/23/24/25-23/24/25-11 |
| Заявления о безопасности | 26-36-24/25-45-36/37-16-7 |
| РИДАДР | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Германия | 2 |
| RTECS | BV8396000 |
| кода HS | 29372900 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H361fd:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению. Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Дегидроэпиандростерон химические свойства, назначение, производство
Описание
Dehydroepiandrosterone(DHEA) is an endogenous steroid hormone that is secreted primarily by the adrenal gland and is the most abundant sex steroid. It is a neurohormone; small quantities of DHEA are produced in the brain and declines in serum and CSF with age. (Knopman and Henderson, 2003). Metabolites of DHEA include androstenedione , which subsequently may be metabolized to testosterone or estrone which is an estradiol precursor. In addition to serving as an intermediate in the biosynthesis of sex steroids, DHEA directly modulates a number of cellular and nuclear receptors.Химические свойства
white fine crystalline powder. There are two crystal forms. Needle-like crystal, melting point 140-141oC; small leaf-like crystal, melting point 152-153oC. Has right-handedness. Soluble in alcohol, ether, benzene, slightly soluble in chloroform, petroleum ether. In case of digitonin to produce precipitation.Вхождение
DHEA is naturally occurring in yam (see Wild Yam, p. 596-597).Использование
Dehydroepiandrosterone (DHEA) is a major C19 steroid produced by the adrenal cortex. It is a major secretory steroidal product of the adrenal gland; secretion progresively declines with aging. May have estrogen-or androgen-like effects depending on the hormonal milieu. Intracellularly converted to androstenedione. It is used in treatment of menopausal syndrome. People living with HIV may be interested in taking DHEA for a variety of reasons, including treatment of depression, increasing bone density, decreasing arterial plaques, improvement of immune function with HIV, increased memory, and increased muscle strength.Определение
ChEBI: Dehydroepiandrosterone is an androstanoid that is androst-5-ene substituted by a beta-hydroxy group at position 3 and an oxo group at position 17. It is a naturally occurring steroid hormone produced by the adrenal glands. It has a role as an androgen, a human metabolite and a mouse metabolite. It is a 17-oxo steroid, an androstanoid and a 3beta-hydroxy-Delta(5)-steroid.Производственный процесс
To a solution of 1 gram of 16-dehydropregnenolon-3β-acetate in 10 ml pyridine is added 0.22 gram of hydroxylamine hydrochloride, and the mixture is allowed to stand at room temperature for four days. One gram of 16dehydropregnenolon-3β-acetate oxime is dissolved in 30 ml of hot dioxane, and then the solution is cooled in an ice bath until about one-half of the dioxane has solidified. Then 1 gram of phosphorus pentachloride is added and the mixture is shaken until all the dioxane has melted. The mixture is maintained at 35°C, for seventy-five minutes, then an excess of ice is added and the solution is again allowed to stand at 35°C. After about thirty minutes, a solution of 5 ml of concentrated hydrochloric acid in 10 ml of water is added, and the mixture is diluted with water, extracted with ether and the ethereal extract washed with dilute sodium hydroxide solution. The ether is removed on a steam bath and the residue is worked up to yield dehydroepiandrosterone.Всемирная организация здравоохранения(ВОЗ)
The World Health Organization has no information further to the above regarding preparations containing prasterone or to indicate that such preparations remain available.Общее описание
DHEA is a major secretory steroidal product of the adrenal gland and is a hormonal precursor to both androgens and estrogens. It can also be synthesized using wild yam or soy, but there is no evidence to show that humans are able to increase DHEA levels by consuming these foods. DHEA and its sulfated metabolite, DHEA-S, are negative modulators of GABAA receptors.Угроза здоровью
An experimental teratogen.Experimental reproductive effects. Questionablecarcinogen with experimental neoplastigenic data. Whenheated to decomposition it emits acrid smoke andirritating fumes.Побочные эффекты
Side effects may include acne, skin rash, GI upset, hirsuitism, hypertension, and increased HDL. In people with HIV, additional side effects may include fatigue, nasal congestion, and headaches.Безопасность
Dehydroepiandrosterone should always be used under the supervision of a medical professional. It is likely safe for people with low DHEA levels to take oral supplements short-term (<6 months) to restore DHEA to normal, but long-term use and doses resulting in high DHEA levels are possibly unsafe. Side effects are often seen with higher doses and longterm use.Дегидроэпиандростерон запасные части и сырье
Дегидроэпиандростерон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8617709210191 | China | 882 | 58 | ||
| +86-029-86333380 18829239519 |
China | 899 | 58 | ||
| 0917-3909592 13892490616 |
China | 9310 | 58 | ||
| +8613004379774 | China | 33 | 58 | ||
| +86-18791163155 +86-13119157289 |
China | 3558 | 58 | ||
| +86-0533-2066820 +8618369939125 |
China | 1009 | 58 | ||
| +86-852-57055271 +8613237129059 |
China | 232 | 58 | ||
| +86-15926415536 +86-15926415536 |
China | 160 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | ||
| +8615387054039 | China | 427 | 58 |