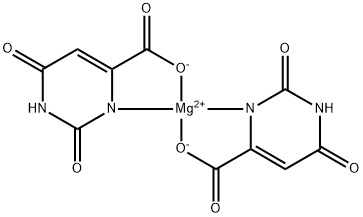
Магния оротат
- английское имяMagnesium Orotate
- CAS №34717-03-8
- CBNumberCB1257706
- ФормулаC10H4MgN4O8
- мольный вес332.47
- EINECS252-167-6
- номер MDLMFCD00039120
- файл Mol34717-03-8.mol
химическое свойство
| температура хранения | -20°C |
| InChI | InChI=1S/2C5H4N2O4.Mg/c2*8-3-1-2(4(9)10)6-5(11)7-3;/h2*1H,(H3,6,7,8,9,10,11);/q;;+4/p-4 |
| ИнЧИКей | MEZKYWVZYGZEHX-UHFFFAOYSA-J |
| SMILES | O=C1NC(=O)C=C2C(=O)[O-][Mg+2]3([O-]C(=O)C4=CC(=O)NC(=O)N34)N12 |
| LogP | -0.928 (est) |
| FDA UNII | GI96W46M5A |
| Код УВД | A12CC09 |
| Система регистрации веществ EPA | Magnesium, bis(1,2,3,6-tetrahydro-2,6-dioxo-4-pyrimidinecarboxylato-.kappa.N3,.kappa.O4)-, (T-4)- (34717-03-8) |
Магния оротат химические свойства, назначение, производство
Описание
Magnesium orotate is a magnesium salt of orotic acid and is poorly soluble in water. It is a source of magnesium and is used as a mineral supplement to treat Mg deficiency. Orotic acid acts as a transporter that carries magnesium into the cells. It also exhibits antioxidant properties, since it is a key intermediate in the biosynthetic pathway of pyrimidines that promotes the synthesis of enzymes which act as free radical scavengers. Experiments investigating the potential cardioprotective actions of orotic acid in pathological heart conditions are still ongoing.Использование
magnesium orotate is used as an adjuvant treatment in congestive heart failure, hyperten-sion, post-operative cardiac status or in type 2 diabetes.Magnesium orotate combines in itself the cardio protective properties of magnesium and orotic acid and leads to the optimisation of the magnesium effect by reducing cellular magnesium loss.
At the same time, animal studies have indicated a protective effect of magnesium orotate with respect to atherosclerotic vascular changes. The efficacy of the combination was superior to the effect of the individual components of magnesium and orotic acid.
Подготовка
For the synthesis of magnesium orotate and due to the low solubility of both magnesium hydroxide and orotic acid, 50 mL H2O were heated up to 70 °C. Half of the magnesium hydroxide (total: 0.125 g, 2.16 mmol, 1 eq.) was added to the warm solution. Subsequently, small amounts of both orotic acid (total: 0.75 g, 4.31 mmol, 2 eq.) and the remaining magnesium hydroxide were added alternatingly. After heating the solution to 90 °C and stirring it for 30 min, it was stored in the fridge for crystallization. After one weekend, 0.70 g (1.46 mmol, 68 %) of a white microcrystalline material could be obtained after filtration and drying.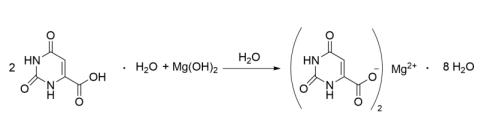
IR, NMR and PXRD analysis (presented in sections S2 and S4) confirmed the product to be magnesium orotate octahydrate.
Магния оротат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-15008457246 +86-15008457246 |
China | 53 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-029-81138252 +86-18789408387 |
China | 3889 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86 18953170293 | China | 2930 | 58 |