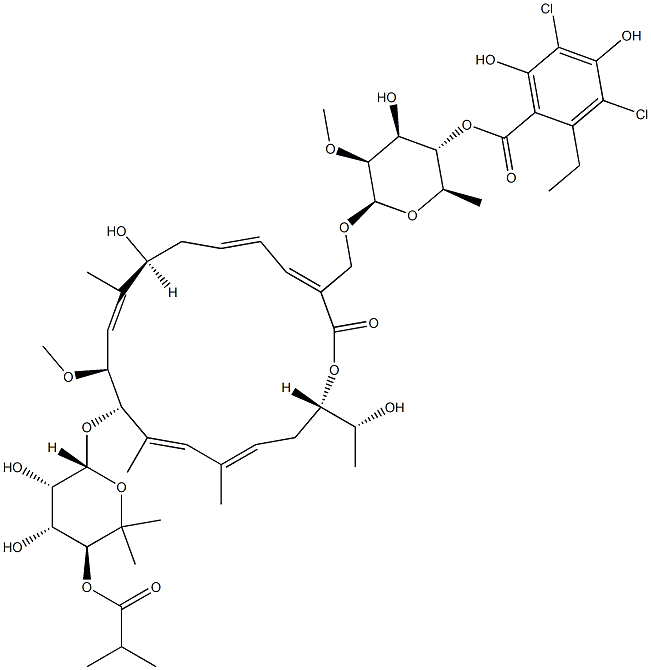
Фидаксомицин
- английское имяFidaxoMicin
- CAS №873857-62-6
- CBNumberCB02582922
- ФормулаC52H74Cl2O18
- мольный вес1058.04
- номер MDLMFCD27976367
- файл Mol873857-62-6.mol
химическое свойство
| Температура плавления | 161 °C |
| Температура кипения | 1046.4±65.0 °C(Predicted) |
| плотность | 1.33 |
| температура хранения | 2-8°C |
| растворимость | Chloroform (Slightly, Heated), DMSO (Slightly, Heated), Methanol (Slightly) |
| форма | powder |
| пка | 5.09±0.35(Predicted) |
| цвет | White to Off-White |
| Биологические источники | (Actinoplanes deccanensis) |
| ИнЧИКей | HRDFANQMSCFNSU-KLTYAODHNA-N |
| Словарь онкологических терминов NCI | OPT-80; PAR-101 |
| FDA UNII | Z5N076G8YQ |
| Словарь наркотиков NCI | fidaxomicin |
| Код УВД | A07AA12 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Фидаксомицин химические свойства, назначение, производство
Описание
Fidaxomicin (OPT-80) was approved by the U.S. FDA in May 2011 for the treatment of Clostridium difficile-associated diarrhea (CDAD), joining metronidazole and vancomycin as drugs recommended for treatment of C. difficile infections (CDI). Fidaxomicin, also known as lipiarmycin and tiacumicin, is an 18-membered macrolide natural product that was first reported in mid-1970s and is produced by fermentation. Fidaxomicin and its primary metabolite OP-1118, which results from hydrolysis of the isobutyryl ester, are narrowspectrum antibacterial agents with activity against gram-positive aerobic and anaerobic organisms, but not against gram-negative organisms. Fidaxomicin and OP-1118 exert their antibacterial activity by inhibiting bacterial RNA polymerase, thereby inhibiting bacterial protein synthesis.The MIC90 (minimum inhibitory concentration to kill 90% of bacteria) for fidaxomicin against C.difficile is 0.125–0.25 μg/mL; OP- 1118 is 4- to 16-fold less potent than the parent compound. Fidaxomicin has been reported to spare native intestinal flora such as Bacteroides spp. and as such, may prevent selection of drug-resistant bacteria. Fidaxomicin is bactericidal to C. difficile and has a low propensity for resistance development with no cross-resistance to existing antibiotics. Fidaxomicin shows minimal systemic absorption following oral administration in preclinical studies and humans.
Использование
Fidaxomicin is a recently marketed antibiotic with a confusing history dating back to its original isolation in 1975. Fidaxomicin is the major analogue of a family of macrocyclic lactones, isolated independently by three different groups from cultures belonging to three different genera (Actinoplanes, Dactylosporangium and Micromonospora) known as lipiarmycin A3, tiacumicin B and clostomicin B1, respectively. Fidaxomicin is a narrow spectrum antibiotic with excellent activity against Gram positive bacteria, notably Clostridium difficile. Fidaxomicin acts in the gastrointestinal tract without undue disruption to gut microbial flora.Определение
ChEBI: An 18-membered macrolide that is a fermentation product obtained from the Actinomycete Dactylosporangium aurantiacum. A narrow spectrum antibiotic used for treatment of Clostridium difficile-related infections.Фармацевтические приложения
Formerly known as difimicin. An 18-membered macrocyclic compound related to the tiacumicin group of antibiotics rather than conventional macrolides. It is active against staphylococci (MIC 0.5–2 mg/L) and most anaerobic Grampositive bacilli and cocci, but Gram-negative bacilli, including Gram-negative anaerobes, are resistant. It is very poorly absorbed when given orally and most interest surrounds its activity against C. difficile (MIC 0.12–0.25 mg/L). Such data as are presently available from clinical trials suggest that it is as safe and effective in the treatment of C. difficile-associated diarrhea as vancomycin.Фидаксомицин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +16313385890 | United States | 3868 | 58 | ||
| +86-021-56795779 +8613651600618 |
China | 1227 | 58 | ||
| +86-0571-28186870; +undefined8613073685410 |
China | 1015 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 025-83697070 | CHINA | 3009 | 60 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| 323-480-4688 | United States | 989 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |