Why did the FDA reject approval of Bardoxolone methyl for chronic kidney disease?
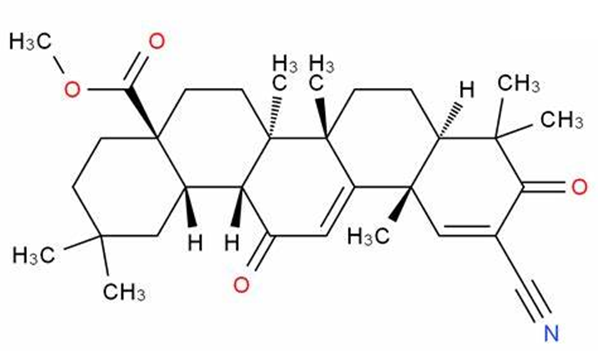
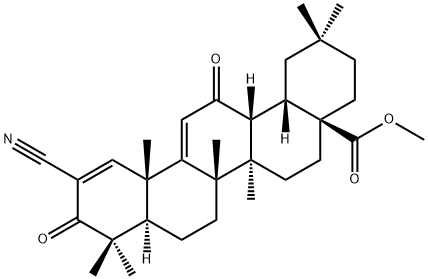
On 25 February 2022, the FDA announced that it could not approve a New Drug Application (NDA) for Bardoxolone methyl for the treatment of Chronic Kidney Disease (CKD) due to Alport Syndrome. The reason for this is that the FDA believes that the data submitted do not demonstrate the effectiveness of bardoxolone in slowing the loss of kidney function and reducing the risk of progression of kidney failure in patients with Alport syndrome. They requested further data to support the efficacy and safety of bardoxolone. The FDA also gave a solution in its letter that would address these issues as long as sufficient evidence of effectiveness from well-controlled studies was provided to show a clinically relevant effect on the rate of loss of renal function in patients with Alport Syndrome. In addition, the company will need to address whether bardoxolone will have a clinically relevant impact on QT intervals and demonstrate that the clinical benefits of bardoxolone outweigh the risks.