What is Zeolite?
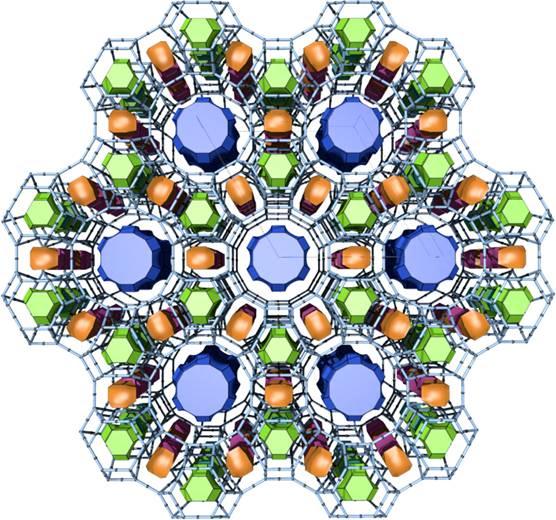
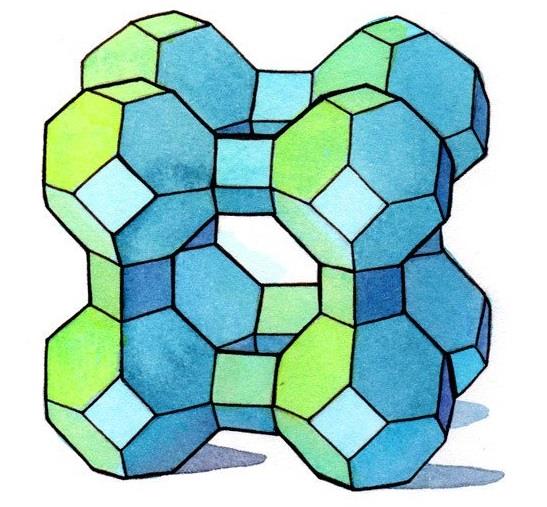
Zeolite, any member of a family of hydrated aluminosilicate minerals that contain alkali and alkaline-earth metals. The zeolites are noted for their lability toward ion-exchange and reversible dehydration. They have a framework structure that encloses interconnected cavities occupied by large metal cations (positively charged ions) and water molecules.
Chemical Composition
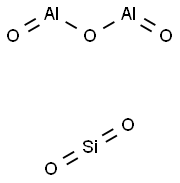
All zeolites are made up of the aluminosilicate framework where silicon and aluminium are tetrahedrally coordinated. Silicon cation and aluminium cations are enclosed by four oxygen anions (O2-). The tetrahedral structure of Si04 and Al04 forms the building block of zeolite.
Zeolite formula is often given as;
Mx/n[AlO2]x.(SiO2)y.mH2O
However, zeolites tend to have different chemical elements in their composition. The formula for zeolite is given in the ratio where,
M = any one metal that could be magnesium, sodium, potassium, lithium, or calcium.
n = valence of the metal cation.
y = number of water molecules in the structure of the zeolite.
y/x = Atomic Si/Al ratio
Types of zeolite
Zeolites are natural minerals that are mined in many parts of the world; most zeolites used commercially are produced synthetically. When developing applications for zeolites, it is important to remember that not all of these minerals are the same.
There are nearly 50 different types of zeolites (clinoptilolite, chabazite, phillipsite, mordenite, etc.) with varying physical and chemical properties. Crystal structure and chemical composition account for the primary differences. Particle density, cation selectivity, molecular pore size, and strength are only some of the properties that can differ depending on the zeolite in question. It is important to know the specific type of zeolite one is using in order to assure that it is appropriate for one's needs.
There are numerous naturally occurring and synthetic zeolites, each with a unique structure. The pore sizes commercially available range from approximately 3 Å to approximately 8 Å. Some of the commercial materials are: A, beta, mordenite, Y, ZSM-5.
The biggest differences between natural and synthetic zeolites are:
Synthetics are manufactured from energy consuming chemicals and naturals are processed from natural ore bodies.
Synthetic zeolites have a silica to alumina ratio of 1 to 1 and clinoptotilite (clino) zeolites have a 5 to 1 ratio.
Clino natural zeolites do not break down in a mildly acid environment, where synthetic zeolites do. The natural zeolite structure has more acid resistant resistant silica to hold it’s structure together. The clino natural zeolite is broadly accepted for use in the agriculutral industry as a soil amendment and as a feed additive.
In 1948, Richard Barrer first produced a synthetic zeolite that did not have a natural counterpart. At approximately the same time, Milton made the first materials that had no natural counterpart such as zeolite A. New natural zeolites are still being discovered, and new synthetic zeolites are being invented in many laboratories around the world.
You may like
Related articles And Qustion
See also
Lastest Price from Zeolite manufacturers

US $6.00/kg2025-04-21
- CAS:
- 1318-02-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $110.00-70.00/kg2025-04-21
- CAS:
- 1318-02-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000