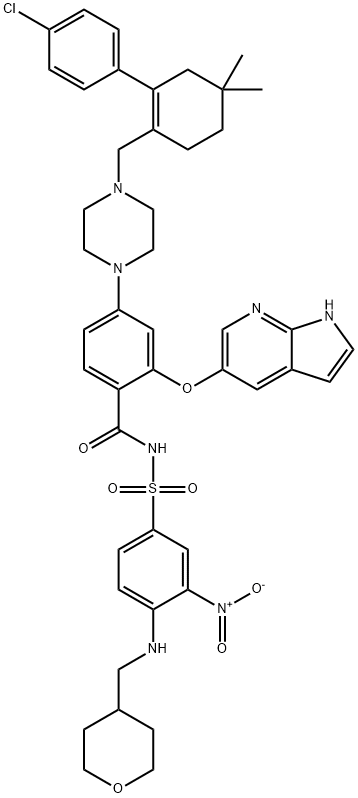
What is Venetoclax/ABT-199?
Feb 10,2020
Venetoclax is a first-in-class, oral, selective BCL-2 inhibitor (BH3-only mimetic). The drug is approved in numerous countries, including those of the EU and in the USA, for the treatment of adults with relapsed or refractory (RR) CLL. The drug arose from research by Abbott Laboratories (now AbbVie) during a collaboration with Genentech and is being codeveloped by AbbVie and Genentech/Roche primarily.[1]
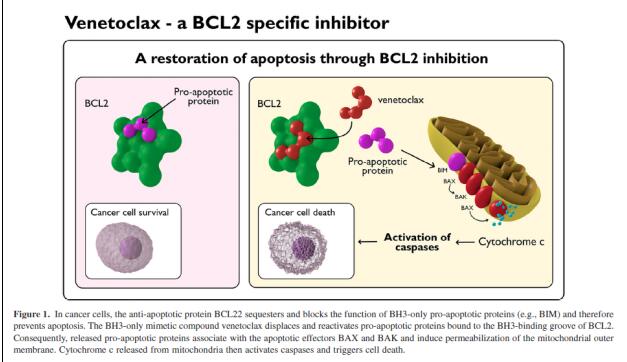
Venetoclax has high affinity for BCL-2, binding to the protein with an affinity more than three orders of magnitude greater than to BCL-XL or BCL-W in vitro. By binding to BCL-2, the drug displaces BCL-2-bound proapoptotic proteins (such as BIM), resulting in the permeabilization of mitochondrial outer membranes, activation of caspases, and restoration of cancer cell apoptosis, with this process requiring the apoptosis regulators BAX or BAK. [2]
Venetoclax displayed cytotoxic activity in various tumour samples/cell lines, including some derived from CLLs, various other NHL subtypes, acute lymphoblastic leukaemias (ALLs), AMLs, chronic myeloid leukaemias (CMLs) and MMs. Notably, the sensitivity of venetoclax correlated with higher BCL-2 expression, with a BCL-2 high status [i.e. BCL-2 gains, BCL-2 amplifications, or the t translocation, which is a notable cause of deregulated BCL-2 expression] and higher BCL-2/MCL-1 ratios being potentially predictive of sensitivity to the drug. [3]
Further preclinical data suggest that sensitivity to venetoclax in T-cell ALL may be determined by ALL subtype/maturation stage, with particular sensitivity observed in early T-cell progenitors and certain immature primary T-cell ALLs, perhaps due to higher levels of BCL-2 expression. [4]
Venetoclax monotherapy or combination therapy with rituximab was an effective treatment, provided durable responses, and had a manageable safety profile in pivotal clinical trials in adults with RR CLL, including in patients with adverse prognostic factors. In combination with 6 cycles of rituximab, venetoclax (fixed 24 months’ treatment) was more effective than bendamustine plus rituximab (6 cycles) in prolonging progression-free survival (PFS) and inducing undetectable minimal residual disease (uMRD) in peripheral blood (PB) and bone marrow (BM), with these benefits sustained during 36 months’ follow-up. [5]
References
1.Besbes S, Mirshahi M, Pocard M, et al. New dimension in therapeutic targeting of BCL-2 family proteins[J]. Oncotarget. 2015, 6(15):12862–71.
2.Seymour J. ABT-199 for chronic lymphocytic leukemia[J]. Clin Adv Hematol Oncol. 2014, 12(10):698–700.
3.Su J, Zhou L, Xia MH, et al. Bcl-2 family proteins are involved in the signal crosstalk between endoplasmic reticulum stress and mitochondrial dysfunction in tumor chemotherapy resistance[J]. Biomed Res Int. 2014, 2014:234370.
4.Vogler M, Dinsdale D, Dyer MJ, et al. ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets[J]. Br J Haematol. 2013, 163(1):139–42.
5.
You may like
Related articles And Qustion
Lastest Price from ABT-199 manufacturers
Venetoclax

US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
ABT-199

US $0.00/kg2025-06-07
- CAS:
- 1257044-40-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg