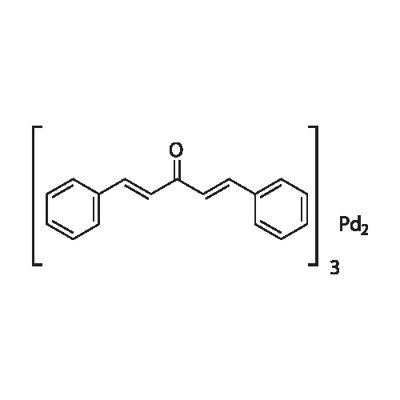
What is Tris(dibenzylideneacetone)dipalladium?
Identification
Product Name: Tris(dibenzylideneacetone)dipalladium
Synonyms: Bis(dibenzylideneacetone)palladium(0)~Pd_2(dba)_3;Tris(dibenzylidenaceTone) dipalladium (O);Tris(dibezylideneacetone)dipalladium;Palladium, tris.mu.-(1,2-.eta.:4,5-.eta.)-(1E,4E)-1,5-diphenyl-1,4-pentadien-3-onedi-;Tris(dibenzylidenaceton)dipalladium;TRIS(BIBENZYLIDENEACETONE) DIPALLADIUM;Tris(dibenzylideneacetone)dipalladium(0), Pd 21.5% min;Tris(dibenzylideneacetone)dipalladium(0), Pd content 22-24%
CAS: 51364-51-3
MF: C51H42O3Pd2
MW: 915.71738
EINECS: 610-654-4
Properties
Melting point 152-155°C
Storage temp. 2-8°C
Solubility Soluble in chlorinated solvents, benzene and THF.
Form Fine Crystalline Powder
Color Purple to black
Water Solubility insoluble
Description
Tris(dibenzylideneacetone) dipalladium (Tris DBA) is used as catalyst for a wide variety of Pd catalyzed reactions including Suzuki coupling, Heck coupling, Negishi coupling, Carroll reaarangement, Trost asymmetric allylic alkylation, Buchwald-Hartwig amination of acryl halides, fluorination of allylic chlorides, arylation of ketones, carbonylation of 1,1-dichloro-1-alkenes, ß-arylation of carboxylic esters, and conversion of aryl and vinyl triflates to aryl and vinyl halides. It is also involved in the synthesis of azepane. Tris DBA is also a novel inhibitor of N-myristoyltransferase-1 with significant antitumor activity.
Reactant involved in:
Reaction type: Cross Coupling Reactions with Arenes, Amination, Buchwald-Hartwig Aminaton, Carbonylation, Mizoroki Heck Coupling Reaction, Stille Reaction, Suzuki-Miyaura Coupling Reaction, Oxidation, Reduction.
• Synthesis of nanosized palladium phosphides upon interaction with white phosphorous
• Preparation of palladium triphenylphosphine carbonyl cluster complexes
• Precursor for synthesis of functionalized multiwalled carbon nanotube-palladium complexes used as catalysts for Heck coupling reactions
• Selective carbon-sulfur bond formation via addition of S-S and S-H bonds to alkynes
Catalyst for:
• Suzuki cross-coupling reactions
• PCN- and PCS-pincer palladium complex catalyzed tandem allylation
• Umicore Precatalysts for Asymmetric and Cross-Coupling Catalysis
• Catalyst for C-C and C-N bond formation especially Suzuki, Heck coupling reaction
Examples of actions:
Palladium catalyzed conversion of aryl chlorides and triflates to nitroaromatics.
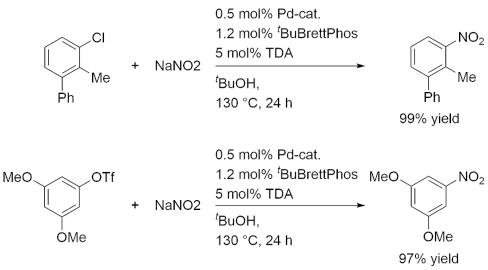
Reference: J. Am. Chem. Soc. 2009, 131, 12898 (DOI: 10.1021/ja905768k)
Site selective sp³ palladium catalyzed direct arylation for a rapid derivatization of heterocyclic compounds.
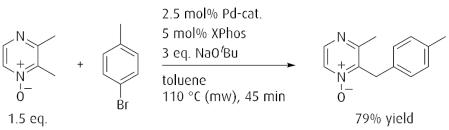
Reference: J. Am. Chem. Soc. 2008, 130, 3266. (DOI: 10.1021/ja710451s)
One-pot ketoindoline synthesis by Palladium catalyzed γ-arylation of β,γ-unsaturated ketones.
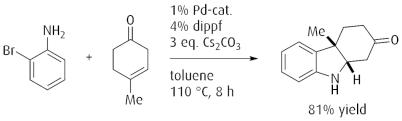
Reference: Angew. Chem. Int. Ed. 2008, 47, 177. (DOI: 10.1002/anie.200704529)
Asymmetric synthesis of indolines by Palladium catalyzed arylation of β,γ-unsaturated ketones.

Reference: Angew. Chem. Int. Ed. 2008, 47, 177. (DOI: 10.1002/anie.200704529)
Suzuki cross coupling of aryl chorides with arylboronic acids.
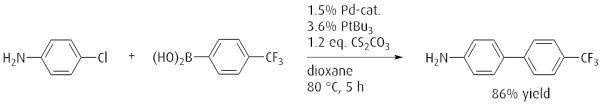
Reference: Angew. Chem. Int. Ed. 1998, 37, 3387. (DOI: 10.1002/(SICI)1521-3773(19981231)37:24<3387::AID-ANIE3387>3.0.CO;2-P)
Efficient method for Suzuki-Miyaura reaction of lithium triisopropyl 2-pyridylborates and aryl chlorides.
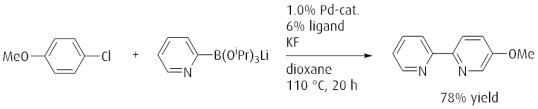
Reference: Angew. Chem. Int. Ed. 2008, 48, 4695. (DOI: 10.1002/anie.200801465)
Palladium catalyzed aminocarbonylation of aryl halides.
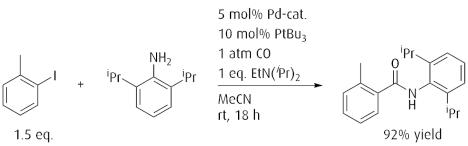
Reference: J. Am. Chem. Soc. 2013, 135, 16841. (DOI: 10.1021/ja4098093)
Related articles And Qustion
Lastest Price from Tris(dibenzylideneacetone)dipalladium manufacturers
US $0.00/KG2025-03-21
- CAS:
- 51364-51-3
- Min. Order:
- 1KG
- Purity:
- 98.00%
- Supply Ability:
- 100KG /month

US $12.00-7.00/g2025-03-07
- CAS:
- 51364-51-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 500