What is theaflavin-3-gallate?
Feb 13,2020
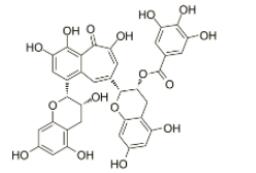
Theaflavin-3-gallate, a black tea theaflavin monomer, formed in the oxidation of epicatechin (EC) and epigallocatechin gallate (EGCG) at the ratio of 1:1, is regarded as the biologically important active component of black tea and provides health benefits. Theaflavin-3-gallate also can be found in the leaves of black tea. Theaflavin-3-gallate act as prooxidants and induce oxidative stress with carcinoma cells more sensitive than normal fibroblasts. Theaflavin-3-gallate reacts directly with reduced glutathione (GSH) in a time- and concentration-dependent manner.
Although ECG and EGCG have already been reported to bind to the surface of PC vesicles, there is little information on the interaction of TFs with simple liposomal PC membranes despite their anticancer, anticholesterol, and hypolipidemic effects observed in experiments using animals and cultured cells. A comparison of the dose-dependent effects of these polyphenols suggested that theaflavin-3-gallate most strongly interacted with PC. Since galloyl groups seem to contribute to the membrane-binding properties of catechins through enhancing hydrophobic interactions, it is not surprising that higher amounts of mono- and di-gallated TFs other than theaflavin (TF, another black tea theaflavin monomer) precipitated after incubation with 3 mm PC vesicles. Comparison of effects of three gallated TFs (theaflavin-3-gallate, theaflavin-3'-gallate, theaflavin-3,3'-digallates) suggests that the 3-position, but not the number, of galloyl group in theaflavin-3-gallate and theaflavin-3,3'-digallates is required for a strong interaction with PC.
In conclusion, theaflavin-3-gallate specifically interacted with PC and with taurocholate-PC micelles resulting in an insoluble complex involving PC and taurocholate. This may be related to the ability of theaflavin-3-gallate to lower the micellar solubility of cholesterol. Since micelles prepared with cholesterol as well as other bile salts have altered lipid membrane fluidity and rigidity, the pattern of theaflavin-3-gallate -induced rearrangement of micelles and the resulting structure would be different from that observed in this study. Electron microscopic analyses by Vermeer et al. confirmed that theaflavin-3-gallate at 0.4 mM, but not three other TFs, affords precipitates consisting of multilamellar vesicles, though they used vesicles consisting of mono-olein, oleic acid, lyso-phosphatidylcholine, cholesterol, and a bile salt mix. Thus, it is possible that such a drastic reconstruction of bile salt-PC micelles occurs during incubation with theaflavin-3-gallate. Further investigations using mimetic models of bile and intestinal micelles are necessary to clarify the inhibitory effects of theaflavin-3-gallate and/or black tea on cholesterol absorption.
References
[1]. Babich H, et al. Theaflavin-3-gallate and theaflavin-3'-gallate, polyphenols in black tea with prooxidant properties. Basic Clin Pharmacol Toxicol. 2008 Jul;103(1):66-74.
You may like
What is Paeoniflorin benefits?
Dec 17, 2024


