What is the use and crystal structure of chromium carbide?
Chromium carbide exists as a gray solid at standard conditions. It is tough and corrosion-resistant. It is also a refractory compound, which means that it retains its strength at high temperatures as well.
Crystal Structure
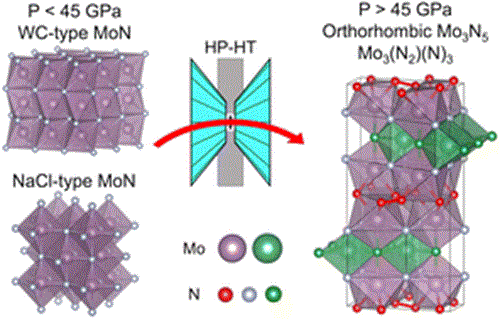
Chromium carbide is the most durable of the three compositions, and has an orthorhombic crystal structure with a microhardness of 2280kg/mm2.
Uses
Chromium carbide is used in a variety of applications. It is used as a wear-resistant coating due to its high hardness and resistance to corrosion. Since it is refractory, it can maintain a good level of wear resistance even at elevated temperatures. Another application is as a grain growth inhibitor. When other types of carbides are produced, chromium carbide is used as an additive to help stop excessive grain growth and improve the toughness of the carbide.
Synthesis
Synthesis of chromium carbide can be achieved through mechanical alloying. In this type of process metallic chromium and pure carbon in the form of graphite are loaded into a ball mill and ground into a fine powder. After the components have been ground they are pressed into a pellet and subjected to hot isostatic pressing. Hot isostatic pressing utilizes an inert gas, primarily argon, in a sealed oven. This pressurized gas applies pressure to the sample from all directions while the oven is heated. The heat and pressure cause the graphite and metallic chromium to react and form chromium carbide. Decreasing the percentage of carbon content in the initial mixture results in an increase in the yield of the Cr7C3, and Cr23C6 forms of chromium carbide.
Safe Handling
Handle in an enclosed, controlled process. Avoid creating dust. Provide adequate ventilation if dusts are created. Avoid breathing dust or fumes. Avoid contact with skin and eyes. Wash thoroughly before eating or smoking.
Safe Storage
Store in a cool, dry area. Store material tightly sealed in properly labeled containers.