What is the synthesis of the nematicide Fluensulfone?
Synthesis of Fluensulfone
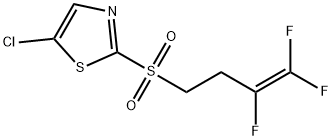
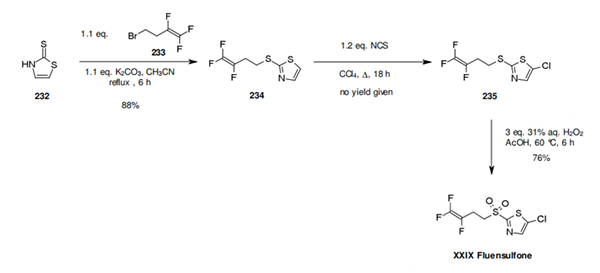
A synthesis of fluensulfone (XXIX) has been described in the original active ingredient patent from Bayer CropScience. The synthesis starts with the alklylation of the 2- mercaptothiazole (232) by 4-bromo-1,1,2-trifluorobutene (233). The thiazole 234 obtained is subsequently chlorinated, and the sulfide function oxidized to the sulphone by hydrogen peroxide in the presence of acetic acid (Scheme 35). In 2002, Bayer CropScience filed a patent for the oxidation of the thioether into the sulphone using Oxone as an oxidant [1.16 eq. Oxone, MeOH-H2O, 20 °C, 2 h (formation of the sulfoxide) then 1.14 eq. Oxone, pH maintained between 8 and 9 with 4N NaOH, 20 °C, 80 min, 92.2% yield, purity 97.6%].
Introduction of Fluensulfone
Fluensulfone is a new nematicide introduced to the market by ADAMA, although originally discovered at Bayer CropScience. It is reported to be effective on plant parasitic nematodes including various species of Meloidogyne. Fluensulfone exerts its action by affecting the mobility and the body posture of nematodes. Those effects are similar to the other well-known insecticide classes such as the organophosphates, carbamates, and the avermectins. However, it has been shown that the mode of action of fluensulfone (XXIX) is distinct from these acetylcholine esterase inhibitors and allosteric modulators of glutamate gated chloride channels of nematodes. Thus, the mode of action remains unknown, although it has been postulated that it may be related to the inhibition of medium-chain acyl-coenzyme A dehydrogenases.