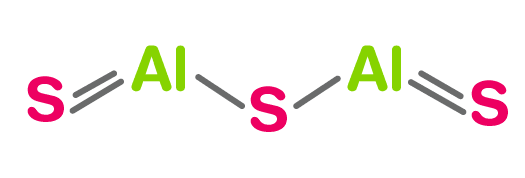
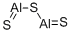What is the name for Al2S3?
Aluminum Sulfide has a chemical formula of Al2S3 and its molar mass is 150.16 g/mol. The compound is formed by two cation Al3+ and three anions S2-. Aluminum sulfide has several crystal structures, some of them are tetragonal, hexagonal, and trigonal.
Uses
Energy storage. A subject of research in the production of aluminum-sulfur batteries, a potentially superior alternative to lithium batteries.
Photovoltaics. Like several similar metal sulfides, aluminum sulfide may be of value for its photovoltaic properties at smaller scales.
Catalysis. Useful for its reactivity in producing several commercially or academically valuable compounds.
Aluminum source. Moderately water and acid soluble source for aluminum.
Safety Hazards of Aluminium Sulfide
Aluminium sulfide is highly toxic in nature. Consequently, it is very dangerous for the health of individuals. Moreover, it can prove fatal if one inhales it. Also, severe damage to the nervous system can take place. This compound can also cause blood damage in the body.
Preparation
Aluminium sulfide can be prepared through a thermite-like reaction between aluminium and sulfur powders.
2 Al + 3 S → Al2S3
The mixture is somewhat difficult to ignite and burns much slower than the classical thermite. The resulting sulfide results in a fused form and is very hard, requiring grinding. If it reaches temperatures greater than 1100 °C, it may melt its way through steel.
You may like
See also
Lastest Price from Aluminium sulfide manufacturers

US $0.00-0.00/KG2024-08-17
- CAS:
- 1302-81-4
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KG

US $15.00-10.00/KG2021-07-02
- CAS:
- 1302-81-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton