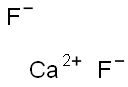What is the Lewis structure of Calcium fluoride?
The Lewis structure of Calcium fluoride (CaF2) consists of a central atom, calcium atom (Ca), and 2 non-metallic fluorine atoms (F). CaF2 is an ionic compound, and the type of bonding is ionic bonding. The CaF2 Lewis structure is shown below:
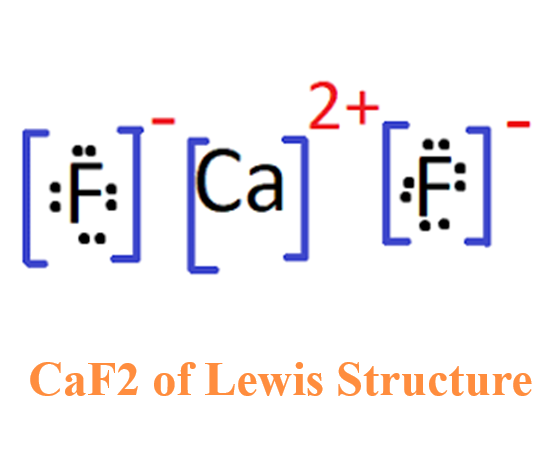
In the Lewis structure drawing of CaF2, the first step is to determine the valence electrons of the atoms. The valence electrons of calcium and fluorine are 2 and 7, respectively, and the electronegativity of fluorine atom is smaller than that of calcium atom, so calcium atom is the central atom, while fluorine atom is the non-central atom. CaF2 is formed by transferring two core electrons (outermost electrons) of calcium to fluorine. Since fluorine atom only needs one more electron to achieve an octet, two fluorine atoms each accept one electron to achieve a stable structure. During this process, calcium atom becomes calcium ion Ca2+, and fluorine atom becomes fluoride ion F-. The oppositely charged ions combine together to form ionic compound calcium fluoride (CaF2).
Related articles And Qustion
Lastest Price from Calcium fluoride manufacturers

US $0.50-0.48/kg2025-07-15
- CAS:
- 7789-75-5
- Min. Order:
- 600kg
- Purity:
- ≥99.5%
- Supply Ability:
- 100 tons

US $25.00/ASSAYS2025-04-21
- CAS:
- 7789-75-5
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt