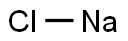What is the charge for NaCl?
NaCl is a neutral species (all chemicals you can put in a bottle are neutral). Of course,the constituent ions, Cl- and Na+ are charged, but as the binary salt there is zero charge.
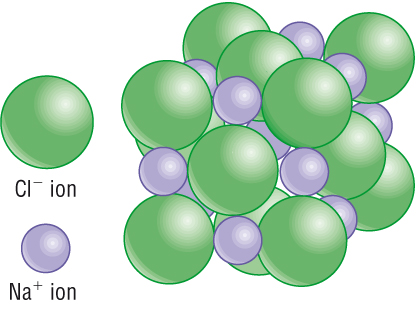
The formation of ions based upon the octet rule is readily seen for the well-known ionic compound, sodium chloride, NaCl. By losing an electron to become the Na+ cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. Chlorine attains a stable octet of 8 outer-shell electrons by gaining 1 electron per atom to produce Cl- ion.
Although it may be a little hard to imagine for a model represented on paper, the six nearest neighbors of each negatively charged Cl- anion are Na+ cations. And the six nearest neighbors of each positively charged Na+ cation are negatively charged Cl- anions. In reality, ions are more accurately represented in an ionic structure as spheres that touch.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium chloride manufacturers

US $0.00-0.00/kg2025-12-13
- CAS:
- 7647-14-5
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 1000kg

US $10.00/KG2025-04-21
- CAS:
- 7647-14-5
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt