What is tert-Butanol?
Chemical Properties
tert-butyl alcohol, also known as tert-Butanol, is a white crystalline solid or colorless liquid (above 77 °F) with a camphor-like odor. It is soluble in water and miscible with alcohol, ether, and other organic solvents. It is highly flammable and easily ignited by heat, sparks, or flames; vapors may form explosive mixtures with air. Fire and explosion may result from contact with oxidizing agents, strong mineral acids, or strong hydrochloric acid. A detection odor threshold concentration of 2,900 mg/m3 (957 ppmv) was experimentally determined by Dravnieks (1974). In a later study, Nagata and Takeuchi (1990) reported an odor threshold concentration 220 ppbv.
Uses
tert-Butyl alcohol is used as a solvent (e.g., for paints, lacquers, and varnishes); as a denaturant for ethanol and several other alcohols; as an octane booster in gasoline; as a dehydrating agent; as a chemical intermediate in the manufacturing of methyl methacrylate; and in the manufacturing of flotation agents, fruit essences, and perfumes.
Butyl alcohols are used as solvents for paints, lacquers, varnishes, natural and synthetic resins, gums, vegetable oils, dyes, camphor, and alkaloids. They are also used as an intermediate in the manufacture of pharmaceuticals and chemicals; in the manufacture of artificial leather, safety glass; rubber and plastic cements, shellac, raincoats, photographic films, perfumes; and in plastic fabrication.
tert-Butanol has been used for a variety of other purposes, including as a dehydrating agent and solvent. tert-Butyl alcohol is used in the production of tert-butyl chloride, tert-butyl phenol, andisobutylene; in the preparation of artificial musk; and in denatured alcohols. tert-Butanol also is used to manufacture methyl methacrylate plastics and flotation devices. Cosmetic and food-related uses include the manufacture of flavors, and, because of its camphor-like aroma, it also is used to create artificial musk, fruit essences, and perfume (HSDB, 2007). It is used in coatings on metal and paperboard food containers (Cal/EPA, 1999) and industrial cleaning compounds, and can be used for chemical extraction in pharmaceutical applications (HSDB, 2007).
Preparation
tert-Butyl alcohol is derived commercially from isobutane as a coproduct of propylene oxide production. It can also be produced by the catalytic hydration of isobutylene, or by a Grignard reaction between acetone and methylmagnesium chloride.
Purification cannot be performed by simple distillation due to formation of an azeotrope with water, although initial drying of the solvent containing large amounts of water is performed by adding benzene to form a tertiary azeotrope and distilling off the water. Smaller amounts of water are removed by drying with calcium oxide (CaO), potassium carbonate (K2CO3), calcium sulfate (CaSO4), or magnesium sulfate (MgSO4), followed by fractional distillation. Anhydrous tert-butyl alcohol is obtained by further refluxing and distilling from magnesium activated with iodine, or alkali metals such as sodium or potassium. Other methods include the use of 4 Å molecular sieves, aluminium tert-butylate, calcium hydride (CaH2), or fractional crystallization under inert atmosphere.
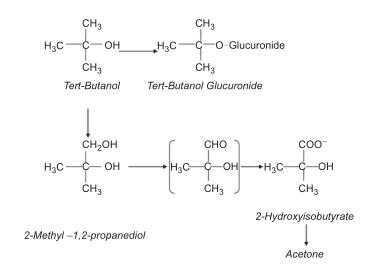
Toxicology
Human exposure may occur due to fuel oxygenate metabolism. Tert-butanol is poorly absorbed through skin but rapidly absorbed if inhaled or ingested. Tert-butanol is irritating to skin or eyes. Toxicity of single doses is usually low but high doses can produce a sedative or anesthetic effect.
Carcinogenicity Test
There were increased incidences of renal tubule adenoma and carcinoma in male rats, transitional epithelia hyperplasia of the kidney in male and female rats, follicular cell adenoma of the thyroid in female mice, and follicular cell hyperplasia of the thyroid and inflammation and hyperplasia of the urinary bladder in male and female mice. In addition, a slight increase in follicular cell adenoma or carcinoma of the thyroid (combined) in male mice may have been related to exposure to t-butyl alcohol. t-Butyl alcohol was inactive on mouse skin as a complete carcinogen or as a tumor promoter.
You may like
Related articles And Qustion
See also
Lastest Price from tert-Butanol manufacturers

US $0.00/kg2025-06-13
- CAS:
- Min. Order:
- 155kg
- Purity:
- 99%
- Supply Ability:
- 20 MT

US $1.00/kg2025-04-21
- CAS:
- 75-65-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt