What is Sulfamic Acid Cleaner?
Introduction
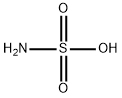
Sulfamic acid is the simplest of the sulfamic acids, consisting of a single sulfur atom covalently bound by single bonds to hydroxy and amino groups and double bonds to two oxygen atoms. Sulfamic acid appears as a white crystalline solid. Irritates skin, eyes, and mucous membranes with low toxicity. It is an acidic cleaning agent, usually for ceramics and metals.

Chemical property
This compound is soluble in formamide and water and slightly soluble in concentrated sulfuric acid, methanol, acetone, and ether. It is commercially produced from fuming sulfuric acid and urea and is classified as a strong inorganic acid. The sulfamic acid with a dilute aqueous solution is stable for longer periods at room temperature. However, a rapid hydrolysis arises at high temperatures. Sulfamic acid is considered less corrosive to metals compared to other strong acid such as hydrochloric acid. As the sulfamic acid retains a scale solubilizing capacity, it is considered ideal for the removal of scale from cooling towers, boilers, coils, condensers, heat exchangers, and a wide range of cooling and heating systems, thereby amassing the efficiency of equipment and plants.
Sulfamic Acid Cleaner
Sulfamic Acid Cleaner contains concentrated crystals that mix with water to make a mild, safe-to-use acid cleaner. Removes dried Portland cement, grout haze, and mortar residue from tile, concrete, and masonry. Safe for use on colored grouts. Removes hard water deposits efflorescence and also etches concrete.
Over the last few years, sulfamic acid has replaced hydrochloric acid as a remover of rust. In homes, it is frequently found as a descaling agent in toilet cleaners and detergents for removing the lime scale. Compared to other strong and most common mineral acids, sulfamic acid has the needed low toxicity, low volatility, and water-descaling properties. It forms water-soluble salts of ferric iron and calcium.
Sulfamic acid provides intrinsic safety; hence, it is preferred over hydrochloric acid for household use. Unlike the most common acids, sulfamic acid does not form chlorine gas if invalidly mixed with hypochlorite-based products.
You may like
Related articles And Qustion
See also
Lastest Price from Sulfamic acid manufacturers

US $1.00/kg2025-04-21
- CAS:
- 5329-14-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/kg2025-03-07
- CAS:
- 5329-14-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons